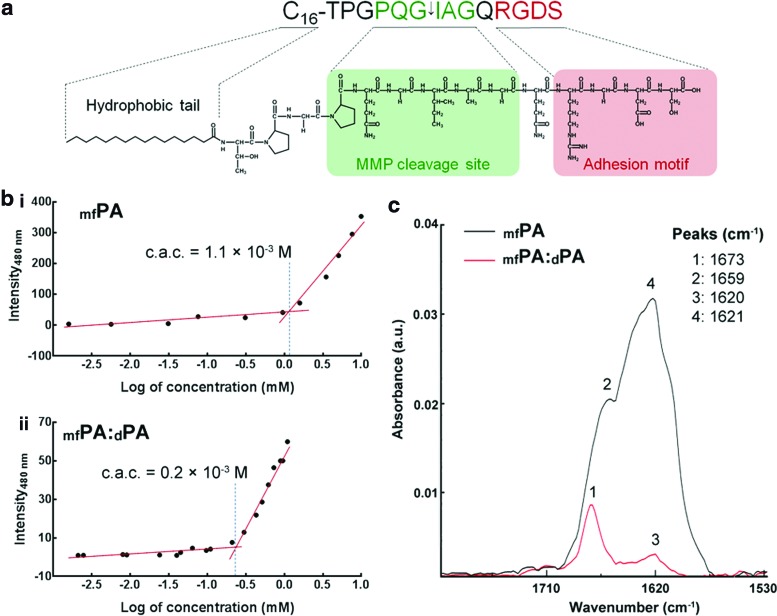
FIG. 1.
The multifunctional C16-TPGPQGIAGQRGDS peptide amphiphile (mfPA). (a) Schematic structure of mfPA comprising a hydrophobic C16 aliphatic chain linked to a hydrophilic 14 amino acid peptide formed by an enzyme-responsive sequence (in green;↓indicates the target site for matrix metalloprotease [MMP]-specific cleavage), followed by the integrin-binding RGDS motif (red). (b) Critical aggregation concentration (c.a.c.) of mfPA in (i) single and (ii) binary systems mixed with diluent PA at a 15:85 mol/mol ratio (mfPA:dPA). (c) Fourier transform infrared (FTIR) spectroscopy analysis of mfPA (black) and mfPA:dPA (red) with corresponding peaks. Color images available online at www.liebertpub.com/tea