Table 3.
Scope of the reaction of various allenes 1 with the sulfonamide 2a.a
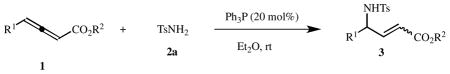
| ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | Time (h) | 3 | Yield (%)b | E/Zc |
| 1 | Me | Et | 12 | 3a | 95 | 93:7 |
| 2 | Et | Et | 24 | 3s | 81 | 94:6 |
| 3 | n-Pr | Et | 48 | 3t | 77 | 94:6 |
| 4 | n-Hex | Et | 72 | 3u | 43 | >99:1 |
| 5 | Ph | Et | 72 | – | – | – |
| 6 | t-Bu | Et | 72 | – | – | – |
| 7 | 2-(4,4-dimethyl)pentyl | Et | 72 | – | trace | N.D. |
| 8 | cyclopentyl | Et | 72 | – | trace | N.D. |
| 9 | Me | Me | 10 | 3v | 93 | 93:7 |
| 10 | Me | PhCH2 | 8 | 3w | 91 | 96:4 |
| 11 | Me | t-Bu | 14 | 3x | 96 | 96:4 |
Reactions of 1 (0.3 mmol) and 2a (0.2 mmol) were performed in Et2O (2 mL) at room temperature.
Isolated yield based on 2.
Determined using 1H NMR spectroscopy.
