Abstract
Purpose
The purpose of the study is to evaluate the CT appearance and pattern of metastatic disease of patients with surgically resected well-differentiated duodenal neuroendocrine tumors who underwent pre-operative dual-phase CT.
Methods
Clinical and pathologic records and CT images of 28 patients (average age 58.0 years) following Whipple procedure were retrospectively reviewed. The size, morphology (polypoid, intraluminal mass or wall thickening, intramural mass), location, CT attenuation in the arterial and venous phases, and the presence of lymph node or liver metastases were recorded.
Results
On CT, 19 patients (67.8%) had neuroendocrine tumors manifested as polypoid or intraluminal masses (38 lesions, multiple tumors in 3 patients), 4 patients (14.3%) had tumors manifested as wall thickening or intramural masses, and in 5 patients (17.9%), the primary tumor was not visualized. Lesions not seen at CT were less than 0.8 cm on pathologic diagnosis. The mean size of polypoid tumors on CT was 1.2 cm (range 0.3–3.8 cm); 24 tumors were 1.0 cm or smaller, and 14 tumors were larger than 1.0 cm. Most lesions were hypervascular in the arterial phase (19/23 patients) with an increase in tumor enhancement in the venous phase in 14 patients (60.9%), decrease in enhancement in 7 patients (30.4%), and no change in enhancement in 2 patients (8.7%). Thirteen patients (46.4%) had metastatic disease from carcinoid tumor, most commonly regional enhancing lymphadenopathy.
Conclusion
Duodenal carcinoid tumors commonly appear as an enhancing mass in either the arterial or venous phases. If a primary tumor is not seen in the duodenum, adjacent enhancing lymphadenopathy can be a clue to the presence of a duodenal carcinoid tumor.
Keywords: Duodenum, Computed tomography, Carcinoid, Neuroendocrine tumor
Gastrointestinal (GI) neuroendocrine tumors are submucosal masses with differentiation in the direction of neuroendocrine cells of the GI tract and comprise 1.2–1.5% of all GI tract neoplasms [1]. Neuroendocrine cells are located throughout the body with two-thirds of neuroendocrine tumors arising in the GI tract, 25% in the lungs, and the remaining 10% in other sites in the body [2]. Neuroendocrine tumors of the GI tract have traditionally been classified according to the embryologic site of origin: foregut, the midgut, or the hindgut; however, it is well known that neuroendocrine tumors of the duodenum and proximal jejunum differ in behavior from tumors arising from the distal jejunum and ileum. The latest World Health Organization (WHO) classification scheme of 2010 classifies neuroendocrine tumors into well-differentiated neuroendocrine tumors (grades 1 and 2), also termed carcinoid tumors, and poorly differentiated neuroendocrine carcinomas (grade 3). In this article, we investigate imaging features of well-differentiated neuroendocrine tumors and use the term carcinoid tumor to refer to well-differentiated neuroendocrine tumors of the GI tract in accordance to the WHO classification scheme [3].
The incidence of neuroendocrine tumors of the GI tract has increased in the last few decades which may in part be due to the increased detection of tumors with wider availability of thin-section multi-detector computed tomography (CT) and endoscopy. For instance, a study based on a national population-based cancer registry in England found the incidence rate of neuroendocrine tumors in the GI tract increased 3- to 4-fold from 1971 to 2006 with an increase of fivefold in the duodenum in men and 6.7-fold in the duodenum in women [2]. This underscores the importance of imaging tests in the primary diagnosis and staging of GI neuroendocrine tumors.
Most duodenal carcinoids are sporadic but may be associated with clinical syndromes such as multiple endocrine neoplasia type 1 (MEN-1) and neurofibromatosis type 1(NF-1) [4]. Two-thirds of duodenal neuroendocrine tumors are gastrinomas and one-third of these are functioning tumors manifesting as Zollinger–Ellison syndrome (ZES). The next most common type (20%) of duodenal neuroendocrine tumors is somatostatinomas. Other more rare types of neuroendocrine tumors are nonfunctioning serotonin-, gastrin-, or calcitonin-producing tumors and gangliocytic paragangliomas [1, 5]. Somatostatinomas are strongly associated with NF-1 as up to 50% of patients with somatostatinomas have NF-1. Somatostatinomas associated with NF-1 are usually found around the ampulla, and they histologically often contain psamomma bodies [5].
From clinical experience, we noticed that duodenal carcinoid tumors occasionally can be very small and may not lose enhancement in the venous phase as is frequently reported. We also noticed that occasionally adjacent enhancing lymphadenopathy may be more prominent than the primary lesion in the duodenum and may be the only sign of a duodenal carcinoid tumor. We wanted to investigate the size, morphology, and enhancement pattern of duodenal carcinoid tumors. Therefore, the objective of this research is to retrospectively evaluate the CT imaging features of duodenal carcinoid tumors and the pattern of metastatic disease on CT with pathologic correlation.
Materials and methods
Study group
At retrospective review of GI neuroendocrine tumors in the pathology archives of patients who underwent pancreaticoduodenectomy (Whipple) procedure accessioned from February 2002 to January 2013, we identified 125 patients with a diagnosis of neuroendocrine tumor. After review of the medical records and radiology database, 96 patients were excluded as the tumor originated outside the duodenum such as the pancreas or the patient did not possess a pre-operative dual-phase CT scan. One patient was excluded as final pathology revealed a high-grade large-cell neuroendocrine carcinoma. This left 28 consecutive patients in our study group who had pre-operative dual-phase MDCT scans with a diagnosis of duodenal or ampullary well-differentiated neuroendocrine tumor. The time from CT scan to surgery ranged from 2 days to 8.5 months with an average time of 1.7 months. The study was performed with the approval of the institutional review board and was compliant with the Health Insurance Portability and Accountability Act. Informed consent was not required.
The study population consisted of 16 women (age range 35–83 years; mean age 59.9 years) and 12 men (age range 40–79 years; mean age 55.7 years). The study population age ranged from 35 to 83 years (mean age 58.0 years, median age 56.5 years). Twenty-two patients were white, five were African-American, and for one patient, race was not specified.
Clinical and pathology data review
Clinical and pathology data were reviewed by two abdominal radiologists, S.D.T. and S.K. Clinical data were reviewed for signs and symptoms at presentation, clinical evidence of excess hormone secretion (peptic ulcer disease, diarrhea, steatorrhea), and documented evidence of NF-1, Zollinger–Ellison syndrome, or MEN-1.
All patients underwent surgical excision (Whipple procedure) of their tumor. Histopathologic and surgical records were reviewed for tumor location and size and evidence of lymph node or liver metastasis by surgical resection.
CT technique and imaging review
Imaging studies were available in all patients. Two body-fellowship trained abdominal radiologists (S.D.T. and S.K.), who have more than 5 and 20 years’ experience at the time of the study, reviewed all images retrospectively with the final interpretation by consensus. The final diagnosis was known at interpretation. CT scans were available for all patients. All patients underwent dual-phase CT with intravenous contrast (Visipaque® 320 or Omnipaque® 350, GE Healthcare, Waukesha WI, USA) with scans obtaining in the arterial and venous phases. A fixed delay of 25–30 s was used for the arterial phase or bolus tracking of the descending aorta. A fixed delay of 60 s was used for the venous phase. All patients were scanned with MDCT with similar scanning protocol of a section thickness of 0.7 mm/0.5 mm for MPR and 3D imaging and reconstructed at 3–5-mm intervals for diagnostic interpretation. Negative oral contrast was routinely given using water or Volumen. To ensure adequate distention of the lumen the CT scan is performed 10 min following the last dose of Volumen or immediately following the last 250 cc of water.
CT scans were reviewed for the presence or absence of a duodenal mass (Table 1). If a mass was present, the number, location, maximum size on axial images, morphologic characteristics (polypoid and intraluminal mass or wall thickening and intramural mass), and contrast enhancement pattern (no enhancement, homogeneous enhancement, or heterogeneous enhancement) were noted. Maximum CT attenuation in the arterial and venous phases of contrast enhancement was also recorded. A region of interest (ROI) was placed in the same area of the mass in both phases and occupying the same percentage of the mass as the lesions were relatively homogeneous. The scans were also evaluated for evidence of lymphadenopathy (enhancing nodes in the peripancreatic, paraduodenal, paraaortic, or mesenteric regions), liver metastases, obstructive biliary dilatation, and adjacent organ invasion. Comparison of location and number of lesions seen on CT was made to the final pathology report from Whipple procedure.
Table 1.
Histology, clinical presentation, and CT appearance of duodenal carcinoid tumors in the study population
| Patient | Histologic type | Signs and Symptoms at presentation | Associated clinical syndrome |
Number of duodenal carcinoids at CT |
CT appearance of primary mass |
Metastases at CT |
|---|---|---|---|---|---|---|
| 1 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | Nonspecific left upper quadrant pain | None | None | Not seen | None |
| 2 | WHO grade I, well-differentiated neuroendocrine carcinoid tumor | Long standing reflux and refractory reflux | None | None | Not seen | None |
| 3 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | Nonspecific reflux and mild anemia | None | 6 | Polypoid intraluminal mass | None |
| 4 | Well-differentiated neuroendocrine neoplasm, somatostatinoma | Abnormal liver function tests | None | 1 | Polypoid intraluminal mass | None |
| 5 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | GI bleeding- melena | None | None | Not seen | None |
| 6 | Low-grade neuroendocrine neoplasm | Asymptomatic, incidentally noted dilated biliary tree on CT for kidney stones | None | 1 | Polypoid intraluminal mass | Enhancing lymphadenopathy adjacent to third portion of the duodenum, liver metastases |
| 7 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | Not specified in medical records | None | 1 | Polypoid intraluminal mass | Four large enhancing lymph nodes adjacent to third portion of the duodenum, larger than primary |
| 8 | Well-differentiated neuroendocrine tumor with features of a somatostatinoma | Weight loss and fatigue | NF-1 | 1 | Wall thickening | Enhancing lymphadenopathy in the root of the mesentery |
| 9 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | Dysphagia-like symptoms | None | 1 | Polypoid intraluminal mass | Small portal caval enhancing lymph node, small hypervascular liver metastases |
| 10 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | Bright red blood per rectum, GI bleed | None | 1 | Polypoid intraluminal mass | None |
| 11 | Three well-differentiated duodenal gastrinomas | Recurrent nausea, abdominal fullness, severe diarrhea, reflux | MEN-1 and ZES | 2 | Polypoid intraluminal mass | Small enhancing lymph node inferior to the second portion of duodenum |
| 12 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | Abdominal pain, diarrhea, vomiting | None | 1 | Polypoid intraluminal mass | Small enhancing adjacent lymph nodes |
| 13 | Low-grade gastrinomas | Prior severe nausea, vomiting, and diarrhea, asymptomatic currently | MEN-1 and ZES | 14 | Polypoid intraluminal mass | Several adjacent enhancing lymph nodes |
| 14 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | Asymptomatic, noted over work up for shortness of breath and occasional right upper quadrant pain | None | None | Not seen | Several adjacent enhancing lymph nodes |
| 15 | Somatostatinoma (low-grade neuroendocrine neoplasm) | Long history of intermittent right upper quadrant pain, also fatigue, and weight loss | None | 1 | Polypoid intraluminal mass | Adjacent enhancing lymph nodes especially portal caval |
| 16 | Well-differentiated carcinoid | Abdominal discomfort | None | 1 | Polypoid intraluminal mass | Small adjacent enhancing lymph nodes |
| 17 | Carcinoid tumor | Weight loss and guaiac positive stools | None | 1 | Polypoid intraluminal mass | None |
| 18 | Well-differentiated neuroendocrine neoplasm | Intermittent epigastric pain, nausea, and vomiting | None | 1 | Polypoid intraluminal mass | None |
| 19 | Carcinoid tumor of the duodenum | Abdominal pain, dyspepsia, diarrhea | None | 1 | Polypoid intraluminal mass | Enhancing lymphadenopathy on prior resection by third portion of the duodenum, but none on CT scan prior to Whipple procedure |
| 20 | Incidental well-differentiated endocrine neoplasm | Painless jaundice | None | 1 | Polypoid intraluminal mass | None, has pancreatic cancer |
| 21 | Small carcinoid tumor | Painless jaundice | None | None | Not seen | None, has pancreatic cancer |
| 22 | Well-differentiated neuroendocrine neoplasm | Abnormal liver function tests | None | 1 | Wall thickening | Small adjacent mildly enlarged enhancing lymph nodes |
| 23 | Well-differentiated neuroendocrine neoplasm | Severe epigastric pain and vomiting | None | 1 | Polypoid intraluminal mass | None |
| 24 | Carcinoid tumor | Weight loss and steatorrhea | None | 1 | Polypoid intraluminal mass | None |
| 25 | Well-differentiated neuroendocrine neoplasm | Anemia | NF-1 | 1 | Polypoid intraluminal mass | None |
| 26 | Well-differentiated neuroendocrine neoplasm | Not specified in the medical records | None | 1 | Wall thickening | None |
| 27 | Well-differentiated neuroendocrine neoplasm | Asymptomatic, incidentally noted after trauma | None | 1 | Wall thickening | Large enhancing portal caval lymph node |
| 28 | Carcinoid tumor (low-grade neuroendocrine neoplasm) | Nausea, vomiting, abdominal pain | NF-1 | 1 | Polypoid intraluminal mass | None |
NF-1 neurofibromatosis type-1, MEN-1 multiple endocrine neoplasia type-1, ZES Zollinger–Ellison syndrome, CT computed tomography, GI gastrointestinal
Results
Clinical-pathologic features
Abdominal pain (n = 8, 28.6%) was the most common complaint at initial presentation. The pain was located in the left upper quadrant in one patient and in the right upper quadrant or epigastric region in three patients; in the four other patients, the location of the pain was not specified. Signs and symptoms of anemia or complaints of GI bleeding were present in four of the 28 patients (14.3%) at initial presentation. Four patients were asymptomatic at presentation with the carcinoid tumor discovered as an incidental findings due to work up for kidney stones (n = 1), shortness of breath (n = 1), trauma (n = 1), and prior history of nausea and vomiting (n = 1). Two patients presented with reflux symptoms (7.1%), and two patients (7.1%) with abnormal liver functions test. The remaining patients presented with weight loss (n = 2), jaundice (n = 2), recurrent nausea and vomiting (n = 1), and dysphagia-like symptoms (n = 1). No patients in our study population presented with duodenal obstruction. The two patients presenting with abnormal liver function tests both presented with biliary obstruction. The initial clinical presentation was not specified in two patients as they were referred to our institution for resection of the tumor.
Two patients (one woman and one man) had MEN-1 syndrome and Zollinger–Ellison syndrome. One presented with recurrent nausea, abdominal fullness, severe diarrhea, and reflux from multiple gastrinomas. The other presented with prior severe nausea, vomiting, and diarrhea from multiple gastrinomas, but was asymptomatic currently.
Three patients (three women) had NF-1. These patients age ranged from 52 to 60 years (mean 55 years). All three of these patients were white. One patient presented with weight loss and fatigue, one with anemia, and one with nausea, vomiting, and abdominal pain. In two of these patients, the duodenal carcinoid tumor was consistent with a somatostatinoma. One patient also had subcutaneous neurofibromas, and one patient had a neurofibroma of the appendix with a focus of neuroendocrine tumor concurrently biopsied at time of Whipple procedure.
Results of pathology and surgical record review at Whipple procedure revealed that the tumor was located in the second portion of the duodenum in 11 patients (39.3%) and in the duodenal bulb in 4 patients (14.3%). Of the 11 patients whose tumors were located in the second portion of the duodenum, 10 had tumors in the peri-ampullary region. Four patients (14.3%) had multiple duodenal carcinoid tumors. The site of carcinoid tumor was not specified on the pathology report in 9 patients (32.1%).
CT features
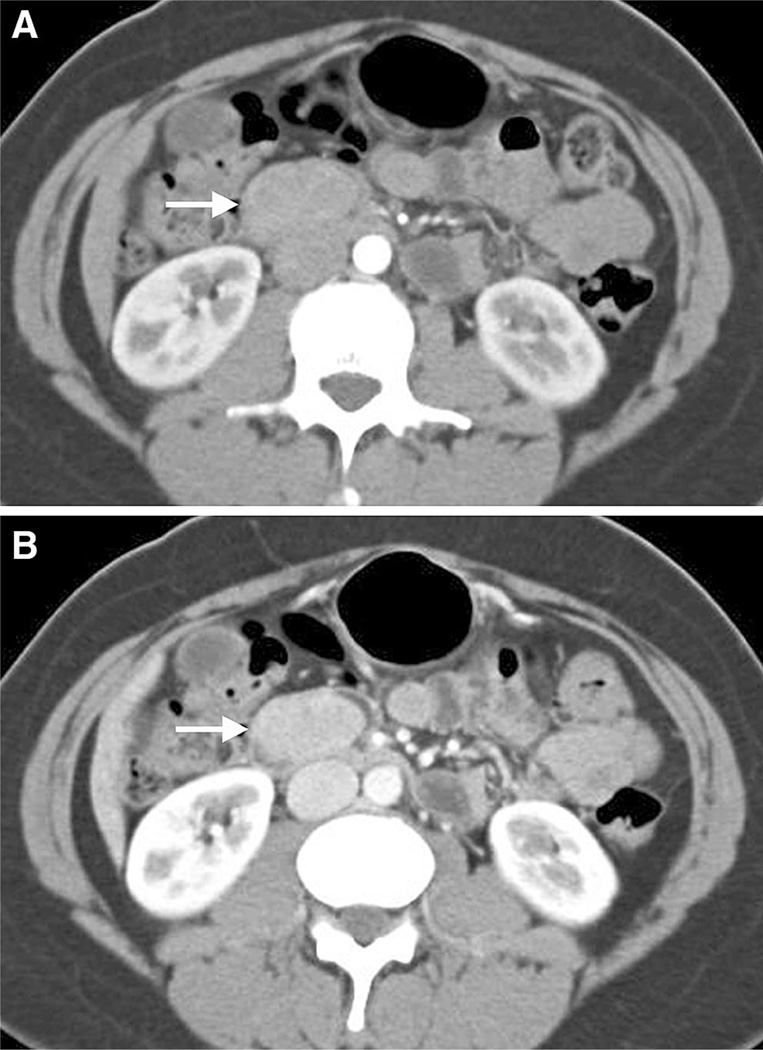
In the 28 patients of the study group, 19 (67.8%) had neuroendocrine tumors manifested as polypoid or intraluminal masses (Fig. 1) and 4 (14.3%) had neuroendocrine tumors that manifested as wall thickening or intramural masses (Fig. 2). No exophytic masses were identified. No patients presented with adjacent organ invasion. In the remainder of the patients (5, 17.9%), the primary tumor in the duodenum was not seen at CT (Fig. 3).
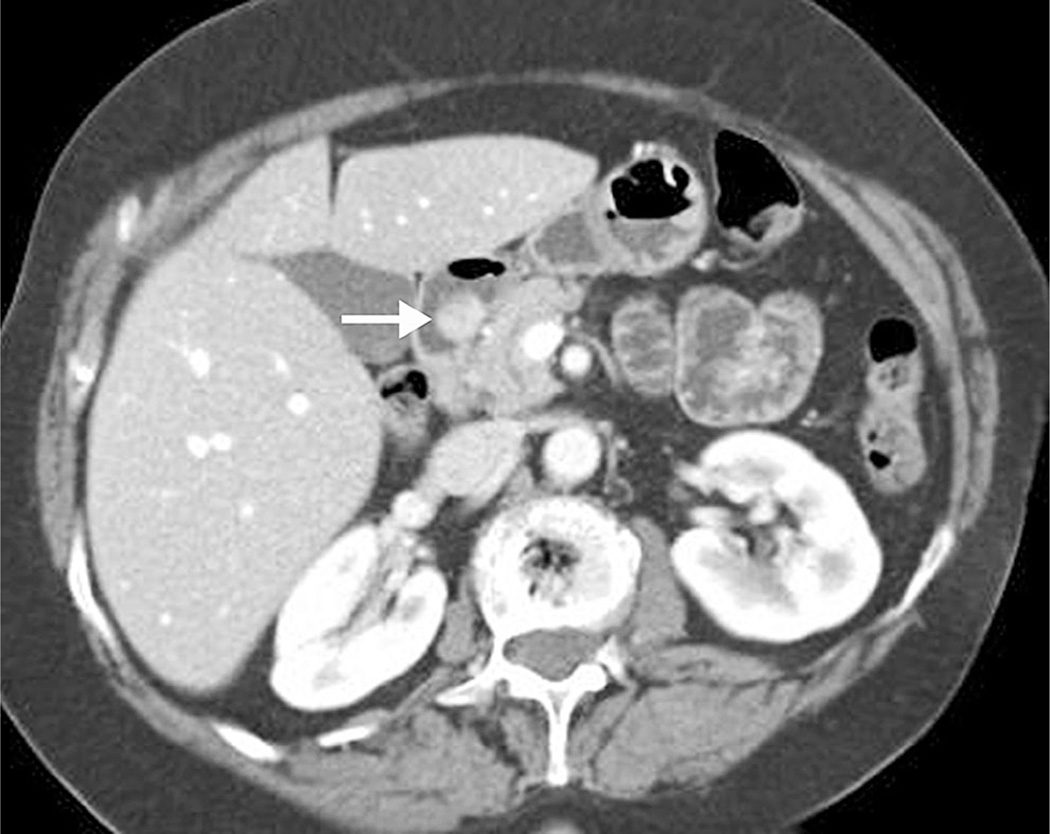
Fig. 1.
74-year-old woman with intermittent epigastric pain, nausea, and vomiting. A well-circumscribed and enhancing polypoid intraluminal mass (arrow) is present in the first portion of the duodenum found to represent a well-differentiated neuroendocrine tumor.
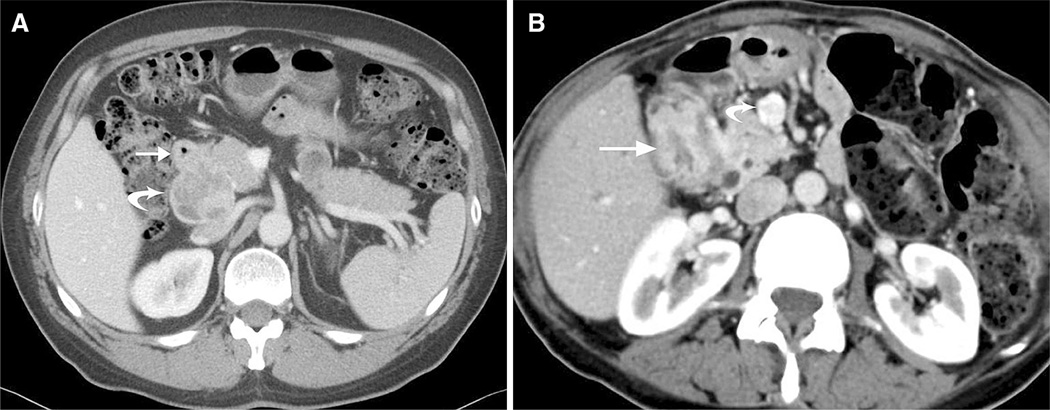
Fig. 2.
A 51-year-old man with incidental neuroendocrine tumor discovered following trauma. Axial CT image of the upper abdomen in the venous phase shows wall thickening of the first portion of duodenum (arrow) without obvious focal mass and a 5.0 × 4.8 cm enhancing and partially necrotic portal caval lymph node (curved arrow). Final pathology revealed a 1.7-cm neuroendocrine tumor of the duodenum and metastatic lymphadenopathy. B 60-year-old female with neurofibromatosis type 1. Axial CT images demonstrate a longer 3.3-cm segment of wall thickening (arrow pathologically proven well-differentiated neuroendocrine tumor with features of a somatostatinoma) and enhancing lymphadenopathy in the root of the mesentery (curved arrow).
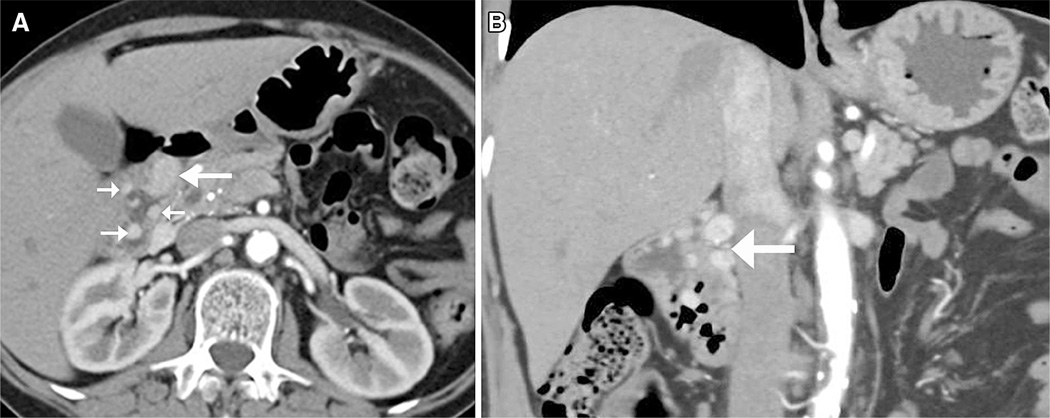
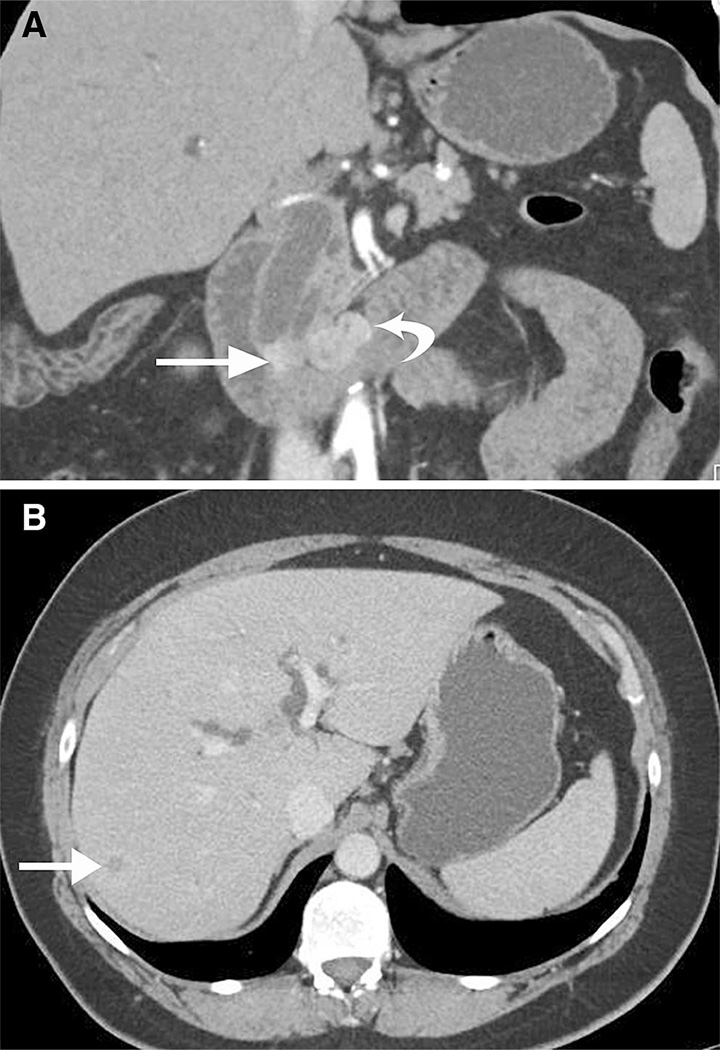
Fig. 3.
58-year-old woman with low-grade neuroendocrine tumor. Coronal CT image in the arterial phase shows an enlarged lymph node (arrow) superior to the third portion of the duodenum without obvious primary lesion in the duodenum. A tiny 0.5-cm duodenal carcinoid tumor was found at histopathologic analysis in addition to metastatic regional lymphadenopathy.
In all but one patient, the masses were homogeneous and well circumscribed without necrosis or calcifications. In one patient, the tumor was heterogeneous containing small cystic areas and calcifications. In the five patients (17.9%) in whom a carcinoid tumor was found at pathologic analysis following Whipple procedure, but not seen at CT, these tumors were all less than 8 mm in size at final pathology. One of the patients in whom a tumor was not seen on CT had pathologically proven metastases to adjacent enhancing lymphadenopathy (Fig. 3), one patient had incidental carcinoid tumor of the duodenum discovered during Whipple procedure for pancreatic adenocarcinoma, and in one patient, the tumor was likely completely excised by endoscopy performed prior to Whipple procedure. In one other patient, the tumor was likely not seen due to inadequate distention of the duodenal lumen and small size of the lesion, and in the another patient, the lesion was not seen on CT even in retrospect with adequate distention of the lumen.
Of the focal polypoid duodenal masses visualized in 19 of the 28 patients (38 discrete lesions), a single lesion was seen in 16 patients (84.2%), two lesions in one patient (5.3%), and greater than two lesions in two patients (10.5%, 6 in one patient and at least 14 in a different patient, Figs. 4, 5). The maximum diameter of polypoid intraluminal masses ranged from 0.3 to 3.8 cm (mean 1.2 cm) with 63.1% of tumors ≤1.0 cm in size (24 masses). Three patients in whom the primary tumor was seen as an area of wall thickening or an intramural mass in the duodenum, also presented with pathologically proven regional adenopathy (Fig. 2).
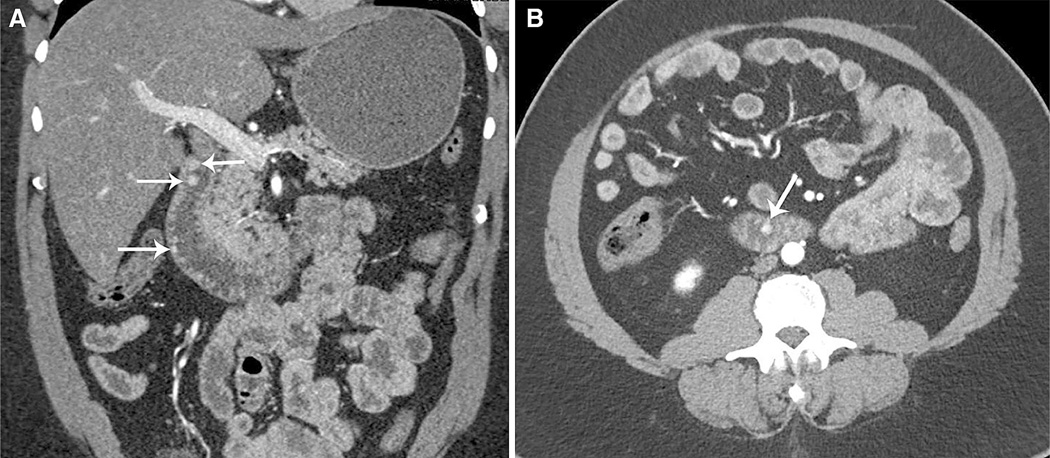
Fig. 4.
57-year-old man with nonspecific reflux and mild anemia and multifocal carcinoid tumors. Coronal (A) and axial (B) CT images in the arterial phase show multiple tiny duodenal well-differentiated carcinoid tumors (arrows). Patient did not have a history of clinical syndrome such as MEN-1 or NF-1.
Fig. 5.
53-year-old woman with MEN-1 and ZES and multifocal low-grade duodenal gastrinomas. Axial (A) and coronal (B) CT images demonstrate a dominant 2.2 × 1.2 cm mass in the duodenum (large arrow in A and B) and multiple additional smaller enhancing polypoid masses (small arrows in A).
Of the neuroendocrine tumors visualized at CT (either as polypoid intraluminal masses or wall thickening and intramural masses), the tumor was located in the second portion of the duodenum in 14 patients (60.9%), in the duodenal bulb in 4 patients (17.4%), and in the third portion of the duodenum in 2 patients (8.7%). Of the 14 patients whose tumors were located in the second portion of the duodenum, 10 (43.5%) had tumors in the peri-ampullary region; two of these patients had NF-1. Six of the peri-ampullary carcinoids produced biliary obstruction with three of these also producing mild pancreatic duct dilation. Seven total patients presented with biliary obstruction with the carcinoid tumor in 6 patients involving the ampulla and in one patient primarily involving the second portion of the duodenum but extending to the ampulla. Three patients (13.0%) had multiple carcinoids: one patient had multiple carcinoids in the bulb and second portion, one patient had multiple carcinoids in the bulb, second, and third portions, and the third patient had multiple carcinoids in the bulb, second, and fourth portions.
In the 23 patients with the neuroendocrine tumor visualized at CT (either as polypoid intraluminal masses or wall thickening intramural masses), 19 patients had enhancing masses and the enhancement pattern was hypervascular in the arterial phase (mean 100.2 HU, range 63–165 HU) with the masses in the remaining 4 patients, isodense to the adjacent duodenum in the arterial phase (mean 74.5 HU, range 55–96 HU). The mean attenuation of all masses in the venous phase was 106.0 HU, range 64–148 HU with an increase in tumoral enhancement in the venous phase in 14 patients (60.9%, Fig. 6), a decrease in tumor enhancement in 7 patients (30.4%), and no change in tumoral enhancement in 2 patients (8.7%, defined as ≤5 HU change from arterial to venous phase).
Fig. 6.
42-year-old woman with severe epigastric pain and vomiting and well-differentiated neuroendocrine neoplasm. Axial CT images in the arterial (A) and venous (B) phases show a well-circumscribed polypoid intraluminal mass in the duodenum (arrows). Hounsfield unit (HU) measurement in the lesion increased from 71 HU in the arterial phase to 112 HU in the venous phase (not shown). There was no evidence of metastatic disease. The presence of an enhancing mass may be sufficient for suggestion of a pre-operative diagnosis of carcinoid tumor.
Thirteen of the 28 patients (46.4%) had evidence of metastatic disease from neuroendocrine tumor at initial presentation by CT and final pathology: Nine patients had enhancing paraduodenal and peripancreatic lymph nodes, two patients had liver metastases and positive peripancreatic lymph nodes (Fig. 7), one patient had tumor with lymph nodes in the root of the mesentery, and one patient had prior resection of a metastatic peripancreatic lymph node but no metastatic lymph nodes at the time of Whipple procedure. In one patient, no primary lesion was seen in the duodenum at CT, and a 0.5-cm lesion was seen at pathology; however, there were several adjacent enlarged lymph nodes positive on final pathology (Fig. 3).
Fig. 7.
49-year-old asymptomatic woman with incidentally noted biliary dilation on CT for kidney stones. Coronal CT image in the arterial phase A shows an enhancing ampullary mass (arrow) resulting in mild pancreatic duct and common bile-duct dilation. Adjacent suspicious, enhancing regional adenopathy (curved arrow in A), and small pathologically proved liver metastasis (arrow in B) are also present.
Imaging features of carcinoids in NF-1
The maximum diameter of the carcinoid tumor in 2 patients with discrete polypoid masses and NF-1 was 2.2 and 2.9 cm, both located in the peri-ampullary region. In one patient with NF-1, the tumor was seen as an area of wall thickening primarily in the second portion of the duodenum but involving the ampulla without a discrete mass. Enhancing lymphadenopathy, which was pathologically proven to be metastatic, was also present in the root of the mesentery in this patient (Fig. 2B). CT showed biliary obstruction in all 3 patients with NF-1. Mild pancreatic duct dilation was also present in one of the patients with NF-1. Vasculopathy, a known although rare complication of NF-1 and which can be seen as fibromuscular dysplasia or aneurysms, was not seen at CT in these 3 patients [6, 7].
Discussion
Duodenal carcinoid tumors comprise approximately 2–3% of all carcinoids of the GI tract [1, 4]. Duodenal gastrinomas are characteristically small (mean size 0.93 cm) with 77% less than 1.0 cm in diameter [8]. Gastrinomas are generally solitary; however, 10–25% of gastrinomas occur in patients with MEN-1 in which the tumors tend to be multiple [1, 4]. Gastrinomas develop in 90% of patients with MEN-1 [5]. In patients with MEN-1, small duodenal gastrinomas can produce large peripancreatic lymph node metastases. These lymph node metastases can clinically mimic a pancreatic primary especially if located near the pancreatic head [9, 10].
Due to the slow-growing nature of the tumor, surgical resection is usually performed as surgery can be curative, and long-term survival is good. Duodenal carcinoid tumors can, however, be highly aggressive with metastases to lymph nodes and liver, the most common sites of metastatic disease. Liver metastases are usually hypervascular similar to the primary tumor and become iso- to hypodense to the liver on delayed phase images. Overall, 5-year survival of GI carcinoid tumors regardless of tumor site and stage approaches 70–80% with worse prognosis in poorly differentiated tumors [4]. If a duodenal carcinoid tumor is suspected, diagnostic imaging including pre-operative CT is helpful to evaluate the extent of the primary tumor including local invasion and tumor staging for any metastatic disease for resection. Multidisciplinary consultation including oncologists, GI surgeons, and pathologists is also valuable to optimize management for each patient.
Demographically, our patient population consisted of middle and older age adults (age range 35–83 years; mean age 58.0 years) and a slight female sex predilection (16 women, 12 men). Racially, most patients were white including the 3 patients with NF-1. This is unusual as duodenal carcinoid in patients with NF-1 as described in the literature occur predominantly in African-American patients [5]. However, similar to published reports, these three patients were all female and had tumors located in the peri-ampullary region often presenting with signs of biliary obstruction [4, 5]. Approximately, 30% of patients with peri-ampullary carcinoid tumors also have NF-1 [4] which is often somatostatinomas [11].
Similar to prior published reports, the clinical presentation of our patients was variable with abdominal pain and GI bleeding the most common presenting complaints. Most duodenal carcinoid tumors are nonfunctioning and therefore present as bowel obstruction, jaundice if peri-ampullary, mass effect or with metastatic disease [4, 5, 11, 12]. The results of our study also demonstrated similar to published reports that duodenal carcinoid tumors were more common in the proximal duodenum seen on CT in the first and second portions in 78.3% in our study [4]. The morphological characteristics were most commonly polypoid or intraluminal masses (67.8%) rather than wall thickening or intramural masses (14.3%).
Our results show that duodenal carcinoid tumors enhance during the arterial phase of intravenous-contrasted enhanced CT and although they do lose contrast enhancement during the venous phase (30.4%) as has often been previously reported [4]; however, in a significant percentage (60.9%), there was an increase in contrast enhancement during the venous phase and no change in contrast enhancement in the venous phase in 8.7% of patients. Early-phase arterial enhancement pattern is an important criterion in distinguishing a duodenal carcinoid tumor from other duodenal masses such as adenocarcinoma which is usually hypovascular, adenomas, or other peri-ampullary masses. The duodenal carcinoid tumor may be more conspicuous in the arterial phase. It is important to recognize that the carcinoid tumor may not lose enhancement in the venous phase and could retain a similar degree of contrast enhancement compared to the arterial phase or even increase in contrast enhancement in the venous phase (Fig. 6). The presence of an enhancing mass may be sufficient for suggestion of a pre-operative diagnosis of carcinoid tumor which can be helpful in surgical planning and in determining prognosis. Esophagogastroduodenoscopy (EGD) or endoscopic ultrasound (EUS) in conjunction with CT findings may also be useful to define these lesions.
The smallest duodenal carcinoid seen at CT in our study was 0.3 cm. The smallest tumor found on CT in a patient with only one lesion was 1.2 cm. In one patient without a history of MEN-1, six well-differentiated duodenal carcinoid tumors were seen ranging from 0.8 to 0.3 cm in size (Fig. 4), and in one other patient with a history of MEN-1 and ZES, one large mass was seen measuring 2.2 cm with multiple additional smaller lesion measuring less than 0.8 cm in size which were consistent with the low-grade gastrinomas on pathologic analysis (Fig. 5). The other patient with MEN-1 and ZES, presented with two tiny (4 and 6 mm) carcinoid tumors on CT in the first and second portions of the duodenum as well as a small enhancing lymph node inferior to the second portion of the duodenum. Sporadic gastrinomas as usually solitary, but gastrinomas in patients with MEN-1 or NF-1 and ZES are usually multiple and tiny (<5 mm in size) and usually located in the proximal duodenum as seen in our patients [5]. Twenty-four lesions in five separate patients measure ≤1.0 cm in size by CT. Modern multi-detector CT with thin slices scanning in both the arterial and venous phases in conjunction with multi-planar coronal and sagittal reconstructions can assist in detecting even these small lesions. Coronal reformatted images may also be helpful in identifying lesions seen as an area of wall thickening. The use of negative oral contrast using water or Volumen coupled with excellent bowel distention can also be helpful in identifying small lesions.
Metastatic disease was seen at initial presentation in 44.8% of our patients in this study. Local lymph node spread was the most common presentation. A carcinoid tumor could therefore be suspected, when local enhancing lymph nodes are present in conjunction with an enhancing duodenal mass (es), but could also be suspected when local enhancing lymph nodes are present with no obvious primary lesion seen in the duodenum at CT. For instance, in one of our patients, an enhancing necrotic lymph node was seen adjacent to the third portion of the duodenum with no primary lesion seen in the duodenum at CT (Fig. 3) [10]. Two patients had small, indeterminate but pathologically proven liver lesions and small enhancing peripancreatic lymph nodes with a polypoid, well-defined, enhancing mass also seen in the duodenum at CT (Fig. 7). It is important to note that metastatic disease can be present in regional lymph nodes even with a small primary tumor size with liver metastases usually appearing late [1, 12, 13]. Therefore, a high index of suspicion for carcinoid tumor should be present if suspicious enhancing regional adenopathy is seen but no obvious primary lesion [10].
Aggressive surgical resection is usually performed due to the slow-growing nature of the tumor and good long-term survival as these tumors tend to progress slowly [4]. Pre-operative CT is helpful to localize extent of primary lesion, evaluate resectability, and for staging for any metastatic disease for complete surgical resection when carcinoid tumor is suspected [4, 14]. Lymph node metastases may be larger than the primary lesion in the duodenum (Figs. 2A, 3, 7) [14]. Size of the primary tumor >2 cm is associated with a higher level of positive regional lymph nodes [13]. Small gastrinomas <1 cm in size can metastasize to regional lymph nodes in 60% of cases [13]. Regional lymph nodes are usually resected with good long-term survival.
Ampullary masses frequently cause biliary obstruction, jaundice, and biliary dilation [8, 11]. This was also seen in our study as of the ten patients with ampullary carcinoid tumors in our study, six resulting in biliary obstruction and three also with mild pancreatic duct obstruction. In contrast to carcinoids tumors located in other portions of the duodenum, ampullary carcinoid tumors are usually more aggressive and can present with metastatic disease regardless of tumor size; therefore, patients are usually treated with a Whipple procedure [15]. Large size of ampullary carcinoids is a poor predictor of metastatic potential [16]. In addition, the CT appearance of an ampullary mass is nonspecific. Although tumor hypervascularity is helpful, ampullary adenomas, carcinomas, and carcinoid tumors may look similar and endoscopy and biopsy or surgical resection would be needed for definitive diagnosis [17].
There are several limitations in our study. First this study is retrospective. Second, although we reviewed carcinoid tumors from the last 10 years resected with Whipple procedure, our sample size was fairly small consisting of 28 patients as several patients were excluded without a pre-operative dual-phase contrast-enhanced CT. Another limitation of this study is that the finding of duodenal carcinoid tumors presenting as an area of wall thickening may be difficult to differentiate from incomplete distention if a focal mass is not seen. We were aware a neuroendocrine tumor was present as this was a retrospective study and a neuroendocrine tumor was seen in the duodenum on final pathology report; however, evaluation of the area in question between the two phases on CT, arterial and venous, if obtained, can be helpful, as a true area of wall thickening secondary to tumor will likely persist between the two phases. It is valuable that the CT protocols on the patients included in this study were generally standardized, being obtained from one institution over 10 years.
In conclusion, carcinoid tumors of the duodenum most often present as a focal polypoid mass, but may present as an area of wall thickening or intramural mass with the primary tumor not well defined. Regional lymphadenopathy may be more pronounced than the primary lesion in the duodenum. The CT features of an enhancing duodenal mass can be suggestive of a carcinoid tumor. Duodenal carcinoid tumors are most common in the proximal duodenum and may present with metastatic disease as evidenced by regional enhancing lymphadenopathy or hypervascular liver lesions.
Footnotes
Disclosures. We have no disclosures.
References
- 1.Chang S, Choi D, Lee SJ, et al. Neuroendocrine neoplasms of the gastrointestinal tract: classification, pathologic basis, and imaging features. Radiographics. 2007;27:1667–1679. doi: 10.1148/rg.276075001. [DOI] [PubMed] [Google Scholar]
- 2.Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563–2569. doi: 10.1038/ajg.2010.341. [DOI] [PubMed] [Google Scholar]
- 3.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th edn. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 4.Scarsbrook AF, Ganeshan A, Statham J, et al. Anatomic and functional imaging of metastatic carcinoid tumors. Radiographics. 2007;27:455–476. doi: 10.1148/rg.272065058. [DOI] [PubMed] [Google Scholar]
- 5.Levy AD, Sobin LH. From the archives of AFIP Gastrointestinal carcinoids: imaging features with clinical pathologic comparison. RadioGraphics. 2007;27:237–257. doi: 10.1148/rg.271065169. [DOI] [PubMed] [Google Scholar]
- 6.Chetty R, Vajpeyi R. Vasculopathic changes, a somatostatin-producing neuroendocrine carcinoma and a jejunal gastrointestinal stromal tumor in a patient with type 1 neurofibromatosis. Endocr Pathol. 2009;20:177–1817. doi: 10.1007/s12022-009-9083-1. [DOI] [PubMed] [Google Scholar]
- 7.Farmakis SG, Han M, White F, Khanna G. Neurofibromatosis 1 vasculopathy manifesting as a peripheral aneurysm in an adolescent. Pediatr Radiol. 2014;44(10):1328–1331. doi: 10.1007/s00247-014-2991-3. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: classification, functional syndromes, diagnosis, and medical treatment. Best Pract Res Clin Gastroenterol. 2005;19(5):675–697. doi: 10.1016/j.bpg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Anlauf M, Perren A, Meyer CL, et al. Precursor lesions in patients with multiple endocrine neoplasia type 1—associated duodenal gastrinomas. Gastroenterology. 2005;128(5):1187–1198. doi: 10.1053/j.gastro.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Anlauf M, Enosawa T, Henopp T, et al. Primary lymph node gastrinoma or occult duodenal microgastrinoma with lymph node metastases in a MEN1 patient: the need for a systematic search for the primary tumor. Am J Surg Pathol. 2008;32(7):1101–1105. doi: 10.1097/PAS.0b013e3181655811. [DOI] [PubMed] [Google Scholar]
- 11.Abraham A, Singh J, Siddiqui G, et al. Endoscopic management of primary duodenal carcinoid tumor. Case Rep Gastroenterol. 2012;6:135–142. doi: 10.1159/000337870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahani DV, Bonaffini PA, Fernández-Del Castillo C, Blake MA. Gastroenteropancreatic neuroendocrine tumors: role of imaging in diagnosis and management. Radiology. 2013;266(1):38–61. doi: 10.1148/radiol.12112512. [DOI] [PubMed] [Google Scholar]
- 13.Mullen JT, Wang H, Yao JC, et al. Carcinoid tumors of the duodenum. Surgery. 2005;128(6):971–978. doi: 10.1016/j.surg.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Pfannenberg AC, Burkart C, Kröber SM, et al. Dual-phase multidetector thin-section CT in detecting duodenal gastrinoma. Abdom Imaging. 2005;30:543–547. doi: 10.1007/s00261-004-0299-8. [DOI] [PubMed] [Google Scholar]
- 15.Poultsides GA, Frederick WA. Carcinoid of the ampulla of Vater: morphologic features and clinical implications. World J Gastroenterol. 2006;12(43):7058–7060. doi: 10.3748/wjg.v12.i43.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishna SG, Lamps LW, Rego RF. Ampullary carcinoid: diagnostic challenges and update on management. Clin Gastroenterol Hepatol. 2010;8(1):e5–e6. doi: 10.1016/j.cgh.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Buck JL, Elsayed AM. From the archives of the AFIP ampullary tumors: radiologic-pathologic correlation. Radiographics. 1993;13:193–212. doi: 10.1148/radiographics.13.1.8426928. [DOI] [PubMed] [Google Scholar]