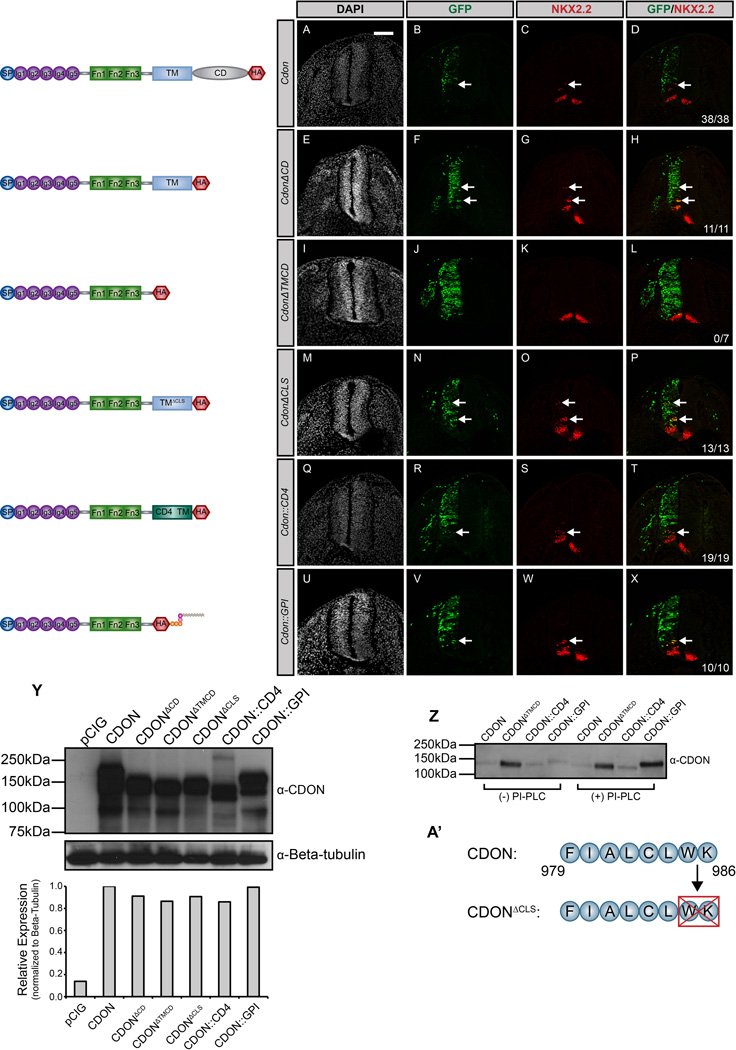
Figure 1. General membrane tethering is sufficient for CDON-mediated HH signaling.
(A–X) Antibody detection of NKX2.2 (red) in forelimb level transverse sections of Hamburger-Hamilton stage 21–22 chicken neural tubes electroporated with Cdon (A–D), CdonΔCD (E–H), CdonΔTMCD (I–L), CdonΔCLS (M–P), Cdon::CD4 (Q–T), and Cdon::GPI (U–X). GFP+ cells (green) designate electroporated cells and DAPI (grayscale) marks nuclei. Merged images are shown on the right, including quantitation of the number of embryos that display ectopic NKX2.2 expression (denoted by arrows). Scale bar, 10µm. (Y) Western blot analysis of COS-7 cell lysates following transfection with the specified constructs and probed with anti-CDON antibody and anti-Beta-tubulin as a loading control. ImageJ quantitation of relative expression levels is expressed as a histogram below the western blots. (Z) Western blot analysis of cell supernatants collected from COS-7 cells transfected with Cdon, CdonΔTMCD, Cdon::CD4, and Cdon::GPI. Cells were untreated (left lanes) or PI-PLC treated (right lanes) prior to supernatant collection. (A’) Schematic illustrating membrane-proximal amino acids that were deleted to generate the CdonΔCLS construct.