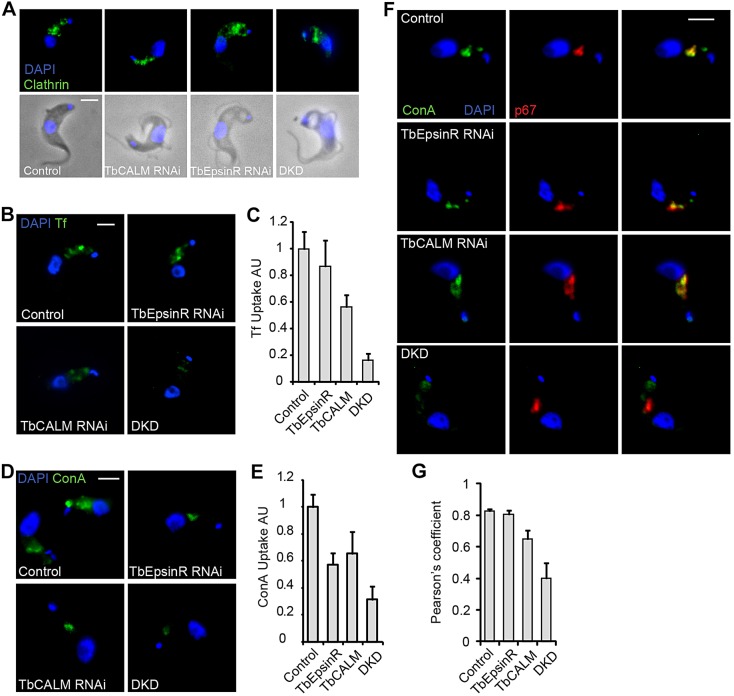
Fig. 6.
Endocytic inhibition following TbCALM and TbEpsinR depletion. (A) Immunofluorescence analysis of clathrin heavy chain distribution (green) in parental (control) and TbCALM, TbEpsinR or double RNAi cell lines (DKD) after 48 h post-induction. Blue indicates DNA DAPI-stain. Note phase-light vacuolar structures reminiscent of the big-eye phenotype observed following clathrin heavy chain depletion (Allen et al., 2003). (B) Uptake of Alexa-Fluor-488-conjugated transferrin in parental (control) and TbCALM, TbEpsinR or double (DKD) RNAi cell lines at 48 h post-induction. (C) Quantification of transferrin as in B. Endocytic inhibition following knockdown of TbEpsinR and TbCALM appears additive, suggestive of some redundancy. Results are mean±s.e.m. (n=50). (D) Uptake of FITC-conjugated ConA in parental and TbCALM, TbEpsinR or double (DKD) RNAi cell lines after 48 h post-induction. (E) Quantification of ConA uptake shown in D. Results are mean±s.e.m. (n=50). Co-depletion of TbCALM and TbEpsinR leads to greater inhibition of ConA uptake than depletion of either protein alone. (F) Lysosomal (p67, red) delivery of ConA in knockdown cell lines after 48 h RNAi induction. Whereas uptake is reduced (D,E), ConA trafficking to the lysosome appears largely unaffected in either TbCALM or TbEpsinR knockdown cells compared to parental control. However, some distension of the p67-positive compartment is apparent following TbCALM depletion. In contrast, ConA uptake appears largely stalled at the flagellar pocket region in the double knockdown. (G) Quantification of ConA and p67 colocalisation following 48 h RNAi induction. Co-depletion of TbCALM and TbEpsinR greatly reduces lysosomal delivery of ConA. Results are mean±s.e.m. (n=50). Scale bars: 2 μm.