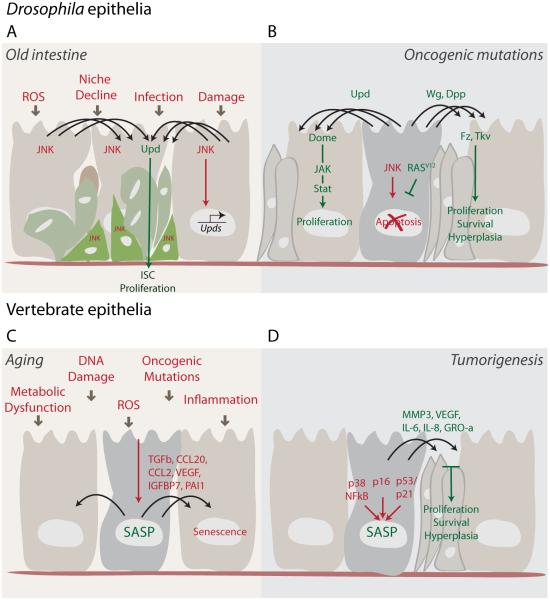
Figure 2. Paracrine signaling promotes tissue degeneration and oncogenic phenotypes with age.
(A) The Drosophila intestinal epithelium deteriorates with age, becoming populated by abnormal and dysplastic cells. These cells accumulate as a consequence of JNK-dependent ISC proliferation and defective differentiation. Different types of damage, including that caused by infection and reactive oxygen species (ROS), create an imbalance of normal signaling events, resulting in the disruption of tissue integrity due to chronic activation of stress signaling and chronic engagement of signaling processes required for tissue repair in the young epithelium (such as Upd secretion).
(B) Oncogenic mutations (such as RasV12) in imaginal disc cell clones can also result in imbalanced paracrine signaling that ultimately causes non-autonomous overgrowth of the tissue. Rasv12 can protect cells from JNK-induced apoptosis. These ‘undead’ cells then promote hyperplasia in the surrounding tissue by continuous secretion of Upd, Wg and Dpp.
(C) In vertebrates, senescent cells induced by different stresses can promote chronic inflammation, which increases during aging due to an accumulation of senescent cells. Some SASP factors (e.g. TGF-β, IGFBP7, PAI-1, CCL2) also induce a senescence response in neighboring cells. Inflammation and the spread of senescence might be the basis for several age-related pathologies, which are characterized by a disruption of normal tissue functions.
(D) Several SASP factors (MMP3, VEGF, IL6, IL8, GRO-α) can also participate in various steps in tumorigenesis, ranging from proliferation to migration and invasion. Thus, senescent cells that accumulate with age and after anti-cancer DNA damaging therapies (chemotherapy, irradiation) can create a milieu for hyperproliferation and tumor growth in the surrounding tissue.