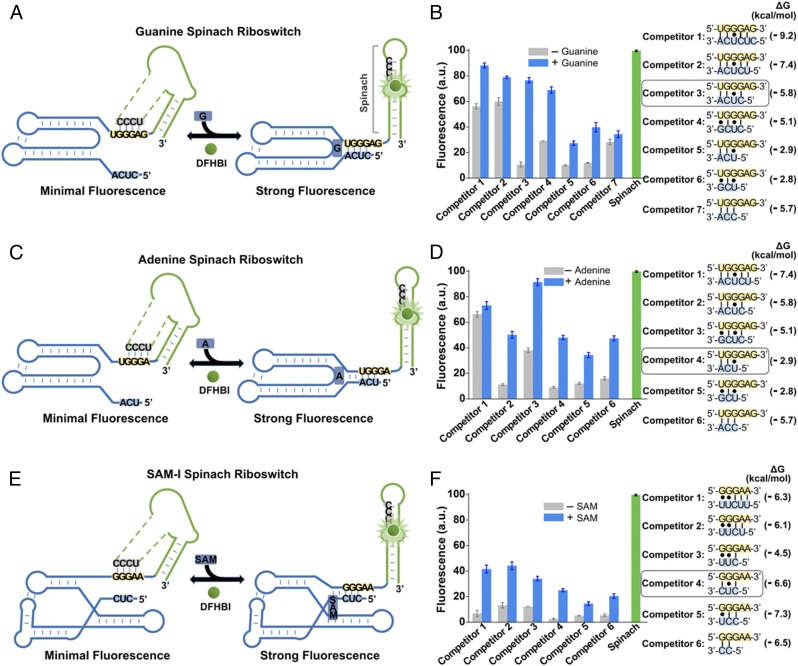
Fig. 6.
Generation of Spinach riboswitches activated by diverse metabolites. (A) Secondary structure and design of the optimal guanine Spinach riboswitch inspired by the natural switching mechanism of the xpt guanine riboswitches. The guanine Spinach riboswitch is generated by swapping Spinach (green) into the expression platform of the xpt guanine riboswitch. Spinach binds and activates the fluorescence of DFHBI only after the target metabolite induces the release of the transducer sequence (gray) from the aptamer domain (blue) so it can bind to the H1 helix in Spinach. The switching sequence and the competitor sequence are highlighted in yellow and blue, respectively. (B) Optimization of the competitor sequence in the guanine Spinach riboswitch. Seven competitor sequences based on the xpt guanine riboswitches (competitors 1–7) were incorporated to transduce guanine binding into a Spinach riboswitch fluorescence signal. The optimal sensor (competitor 3) displayed a 6.2-fold increase in fluorescence signal upon the addition of 10 µM guanine. Shown are mean and SEM values of three independent replicates. The Gibbs free energy change (∆G) of hybridization events between each competitor sequence and the switching sequence was calculated using Mfold online software. (C) Secondary structure and design of the optimal adenine Spinach riboswitch inspired by the switching mechanism of the xpt adenine riboswitches. The adenine Spinach riboswitch is generated by swapping Spinach (green) into the expression platform of the xpt adenine riboswitch. Spinach binds and activates the fluorescence of DFHBI only after the target metabolite induces the release of the transducer sequence (gray) from the aptamer domain (blue) so it can bind to the H1 helix in Spinach. The switching sequence and the competitor sequence are highlighted in yellow and blue, respectively. (D) Optimization of the competitor sequence in the adenine Spinach riboswitch. Six competitor sequences based on the xpt adenine riboswitch (competitors 1–6) were incorporated to transduce adenine binding into a Spinach riboswitch fluorescence signal. The optimal sensor (competitor 4) displayed a 4.3-fold increase in fluorescence signal upon the addition of 100 µM adenine. Shown are mean and SEM values of three independent replicates. The Gibbs free energy change (∆G) of hybridization events between each competitor sequence and the switching sequence was calculated using Mfold online software. (E) Secondary structure and design of the optimal SAM-I Spinach riboswitch inspired by the natural switching mechanism of the yitJ SAM-I riboswitches. The SAM-I Spinach riboswitch is generated by swapping Spinach (green) into the expression platform of the yitJ SAM-I riboswitch. Spinach binds and activates the fluorescence of DFHBI only after the target metabolite induces the release of the transducer sequence (gray) from the aptamer domain (blue) so it can bind to the H1 helix in Spinach. The switching sequence and the competitor sequence are highlighted in yellow and blue, respectively. (F) Optimization of the competitor sequence in the SAM-I Spinach riboswitch. Six competitor sequences from the yitJ SAM-I riboswitch (competitors 1–6) were incorporated to transduce SAM binding into a Spinach riboswitch fluorescence signal. The optimal sensor (competitor 4) displayed an 8.8-fold increase in fluorescence signal upon the addition of 100 µM SAM. Shown are mean and SEM values of three independent replicates. The Gibbs free energy change (∆G) of hybridization events between each competitor sequence and the switching sequence was calculated using Mfold online software.