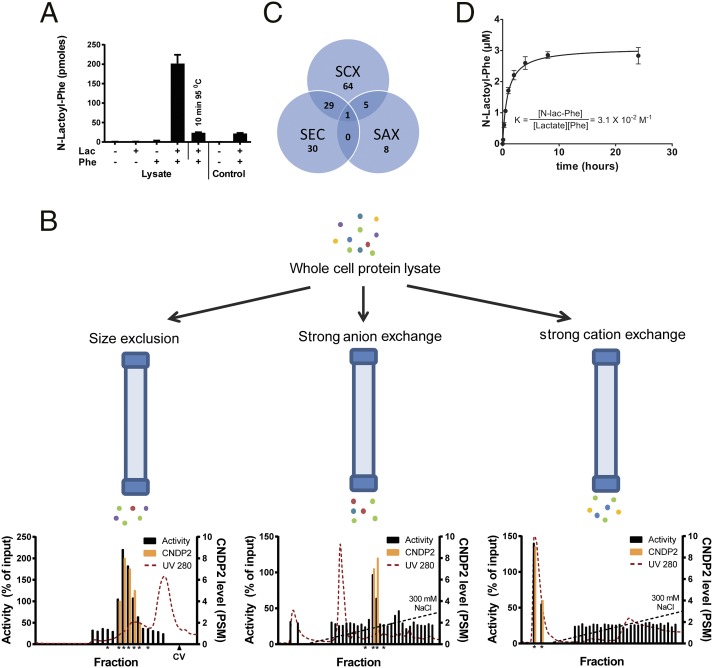
Fig. 4.
N-lac-Phe is formed by CNDP2. (A) N-lac-Phe is formed in the presence of Phe, lactate, and intact protein. Whole-cell lysate or control buffer [25 mM Tris⋅HCl (pH 7.4)] was incubated for 30 min at 37 °C in the presence or absence of 10 mM substrates. N-lac-Phe formation was determined by LC/MS and is expressed in arbitrary units. Data are presented as mean (n = 3) plus SD. (B) Whole-cell lysate was fractionated in parallel on three different columns. Enzyme activity was assessed by incubation with 10 mM lactate and Phe (30 min, 37 °C) and is normalized to the activity in unfractionated whole-cell lysate. The levels of CNDP2 were determined in active fractions and neighboring inactive control fractions (all marked by an asterisk) using LC/MS proteomics and are expressed as a peptide spectrum match (PSM) (n = 1). CV, column volume. (C) Although multiple proteins coeluted with enzyme activity for each single fractionation, only a single protein, CNDP2, coeluted with activity in all three fractionations. SAX, strong anion exchange; SCX, strong cation exchange; SEC, size exclusion chromatography. (D) Human recombinant CNDP2 (1 μg) was incubated (37 °C) with 10 mM lactate and Phe in 25 mM Tris⋅HCl (pH 7.4) containing 0.1 mM MnCl2. N-lac-Phe levels were determined by LC/MS and are expressed as arbitrary units. Data are presented as mean (n = 3) plus SD. Additional enzyme kinetics are presented in Figs. S5, S8, and S9.