The 2008 WHO classification described 36 different types of mature B-cell neoplasms, encompassing provisional entities and subtypes.1 Histological and molecular findings, as well as profound differences in clinical behavior, warrant such a detailed classification. Moreover, even within single lymphoma entities a considerable heterogeneity in disease presentation and outcome among different patients is regularly observed. Hence, a more comprehensive characterization of the patient as well as disease becomes crucial to tailor a “personalized” approach based on the specific features of each of our patients.
An illustrative example is mantle cell lymphoma (MCL). MCL was first recognized as a separate entity as “centrocytic type” in the Kiel classification and subsequently renamed “mantle cell lymphoma”, but was generally not accepted before the 1994 REAL classification.2,4 With a 36-month median overall survival (OS) it was the lymphoma subtype with the worst long-term prognosis, lacking both the prolonged survival of the indolent lymphomas and the curative potential of the aggressive ones.5 Since then, substantial progress has been made based on an improved diagnostic accuracy by the detection of the chromosomal translocation t(11;14) and the resulting cyclin D1 overexpression. Accordingly, its prognosis, formerly recognized as uniformly dismal, has nowadays changed into a spectrum of highly heterogeneous clinical scenarios, irrespective of patient age at presentation.
Reviewing our daily experience, there are certainly young MCL patients who initially respond to cytarabine-containing regimens followed by autologous stem-cell transplantation (ASCT), but who rapidly progress with chemorefractory disease shortly after. On the other hand, some young MCL patients are alive without evidence of lymphoma ten years after ASCT. Similarly, we recollect elderly patients responding to conventional immunochemotherapy and relapsing six months after end of rituximab maintenance. Other individuals may present with a long history of indolent MCL, yet finally, after years of ‘watch and wait’, transform into highly aggressive disease, but promptly respond to targeted approaches like the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib.6 Thus, especially MCL represents the paradigm of a neoplasm in which knowledge of clinical and molecular prognostic factors is critical and may lead to an individualized, and thus much more appropriate, therapeutic approach.7
Until the end of the 1990s, no specific treatment for MCL was known, and conventional polychemotherapy regimens applied in other lymphomas, such as CHOP, MCP, and later on the monoclonal antibody rituximab, were applied.7 More recently, the clinical criteria of “biological” age and comorbidities are being used for individualized treatment approaches, e.g. recommending the more effective high-dose cytarabine-containing regimens and ASCT in young and fit patients.8–10 However, it is well known that a minority of clinically suspected “indolent” MCL cases (mainly characterized by splenomegaly and bone marrow involvement, but absence of lymphadenopathies) do not require such an aggressive approach, and may be safely followed by a ‘watch and wait’ approach.11 Moreover, the excellent outcome of a subgroup of classical MCL characterized by ‘low-risk’ MCL International Prognostic Index (MIPI) and low Ki-67 proliferative index raises the question as to whether such patients might be spared from more intensive (and toxic) treatments without jeopardizing their prognosis.12, 13 Finally, the recent availability of many targeted drugs, such as immunomodulatory compounds (lenalidomide), inhibitors of BTK (ibrutinib) and phosphoinositide 3-kinase (PI3K), or selective BCL2 inhibitors (ABT-199) represent a new challenge of personalized medicine in MCL as the established prognostic markers might be no longer valid for such targeted approaches.6,14–15 Thus, despite the high response rates of these molecules, currently there is no consensus on when, how (single vs. combined approaches) and in whom these approaches should be preferred.
Hence, a deeper knowledge of clinical or biological features predicting sensitivity or refractoriness to specific drugs is urgently needed, also to justify the sometimes expensive approaches in a rational therapeutic algorithm of these new compounds in a “real life” therapeutic scenario. Currently, in the clinic, routine selection of optimal treatment is mainly based on clinical risk factors, symptoms and tumor load. Among many promising prognostic tools, the clinical aggressiveness determined by MIPI score and Ki-67 proliferative index, as well as post-treatment evaluation of minimal residual disease (MRD), represent the only predictors validated in large clinical trials.16–18 All of these are able to stratify patients into different risk classes and should, therefore, be applied in future clinical trials to allow a tailored therapy of MCL.
However, the wide variety of the clinical course cannot be reliably predicted only on the basis of these simple prognostic markers. Over recent years, many new molecular pathways implemented in tumor survival, aggressiveness and treatment refractoriness have been identified.19 Thus, SOX 11 negativity has been initially considered as an indicator of a more indolent subtype.20 On the other hand, in the Scandinavian series, these patients had a more aggressive clinical course due to frequent underlying p53 alterations.21 However, despite extensive basic research and clinical advances, molecular targeted therapies are still limited in this field. For the moment, there is still no reliable translation of biological data into the context of clinical patient care, and the armamentarium of prognostic markers and targeted therapies does not yet allow a personalized strategy in the majority of cases. Thus, current studies should aim to identify predictors of responsiveness to develop a more rational use of the available new drugs. Unfortunately, such biological characterization of the single patient and subsequent treatment tailoring requires complex study designs using mutational and gene expression analyses, based on innovative laboratory techniques such as high-throughput sequencing. At the moment, such analyses are reserved for investigational studies due to their high costs and limited availability. However, it is likely that, in the near future, as costs of the molecular diagnostics will decrease significantly and more evidence as to their usefulness becomes available, these tools will sooner or later become an essential part of clinical routine.
Current prognostic markers, especially MIPI and Ki-67, are easily applicable in routine clinical practice (in Figure 1 a rational therapeutic algorithms is suggested).12 For the moment, considering the time needed, the resources, money savings and improved efficacy of personalized medicine, a rational usage of such predictors may be beneficial even for the general health system, although a detailed analysis of costs and benefits has not yet been performed in clinical trials. Moreover, the current therapeutic scenario in MCL will be completely transformed by the introduction of numerous of the aforementioned new drugs in the near future. Thus, it will be even more important to identify individual patients, especially taking advantage of distinct compounds; moreover, the current continuous administration of these molecules (until disease progression), and the limited knowledge about their long-term toxicities, hamper their general application and a more personalized approach is needed. Given this, it is essential to define a rational therapeutic algorithm for these new compounds based on patient risk profile and individual prediction of response. In this context, routine application of high-throughput molecular diagnostics will form the basis for a new concept of tailored medicine applicable in clinical care.
Figure 1.
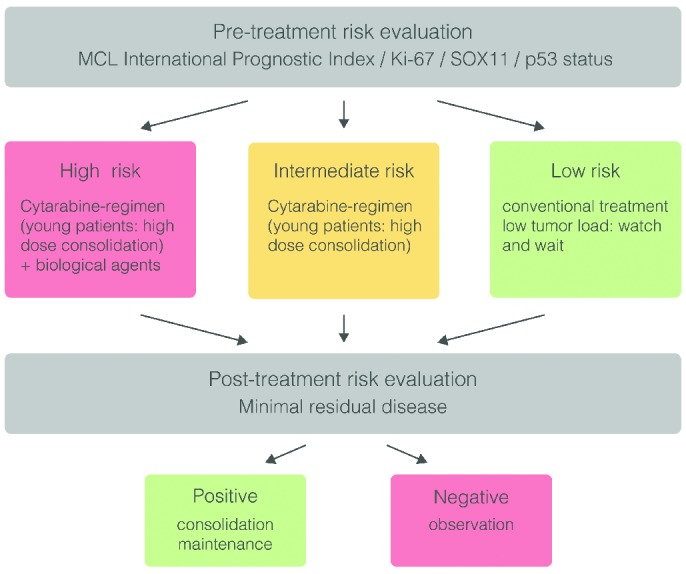
Suggested personalized treatment strategy according to risk stratification in mantle cell lymphoma (MCL).
In conclusion, personalized medicine is the next logical advance in the treatment of MCL, potentially providing both clinical and economical benefits. However, to move from a “one size fits all” to a tailored therapeutic approach and applying the most effective treatment in the individual patient, it is crucial to understand the underlying molecular mechanisms of cancer. The new high-throughput sequencing techniques are paving the way for such a molecular “classification”, but more importantly, this approach will allow an individually optimized treatment strategy, especially in such a heterogeneous disease as MCL.
Footnotes
Financial and other disclosures provided by the author using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are available with the full text of this paper at www.haematologica.org.
References
- 1.World Health Organization, Swerdlow SH, International Agency for Research on Cancer. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: Internat. Agency for Research on Cancer; 2008. 439 S. [Google Scholar]
- 2.Lennert K, Stein H, Kaiserling E. Cytological and functional criteria for the classification of malignant lymphomata. Br J Cancer Suppl. 1975;2:29–43. [PMC free article] [PubMed] [Google Scholar]
- 3.Banks PM, Chan J, Cleary ML, et al. Mantle cell lymphoma. A proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol. 1992;16(7):637–640. [DOI] [PubMed] [Google Scholar]
- 4.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361–1392. [PubMed] [Google Scholar]
- 5.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27(4):511–518. [DOI] [PubMed] [Google Scholar]
- 6.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–565. [DOI] [PubMed] [Google Scholar]
- 8.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677–2684. [DOI] [PubMed] [Google Scholar]
- 9.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermine O, Hoster E, Walewski J, et al. Alternating Courses of 3× CHOP and 3× DHAP Plus Rituximab Followed by a High Dose ARA-C Containing Myeloablative Regimen and Autologous Stem Cell Transplantation (ASCT) Increases Overall Survival When Compared to 6 Courses of CHOP Plus Rituximab Followed by Myeloablative Radiochemotherapy and ASCT in Mantle Cell Lymphoma: Final Analysis of the MCL Younger Trial of the European Mantle Cell Lymphoma Network (MCL net). Blood. ASH Annual Meeting Abstracts 2012;120:151. [Google Scholar]
- 11.Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27(8):1209–1213. [DOI] [PubMed] [Google Scholar]
- 12.Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma international prognostic index in randomized trials of the European mantle-cell lymphoma network. J Clin Oncol. 2014;32(13): 1338–1346. [DOI] [PubMed] [Google Scholar]
- 13.Dreyling M, Ferrero S, Vogt N, Klapper W; European Mantle Cell Lymphoma Network. New paradigms in mantle cell lymphoma: is it time to risk-stratify treatment based on the proliferative signature? Clin Cancer Res. 2014;20(20):5194–5206. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Galán P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Wang M, Romaguera J. Current regimens and novel agents for mantle cell lymphoma. Br J Haematol. 2014;167(1):3–18. [DOI] [PubMed] [Google Scholar]
- 16.Dreyling M, Geisler C, Hermine O, et al. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii83–92. [DOI] [PubMed] [Google Scholar]
- 17.Pott C, Hoster E, Delfau-Larue MH, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood. 2010;115(16):3215–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pott C, Macintyre E, Delfau-Larue MH, et al. MRD Eradication Should be the Therapeutic Goal in Mantle Cell Lymphoma and May Enable Tailored Treatment Approaches: Results of the Intergroup Trials of the European MCL Network. Blood. ASH Annual Meeting Abstracts 2014. n.147. [Google Scholar]
- 19.Beà S, Valdés-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci USA. 2013;110(45):18250–18255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernàndez V, Salamero O, Espinet B, et al. Genomic and gene expression profiling defines indolent forms of mantle cell lymphoma. Cancer Res. 2010;70(4):1408–1418. [DOI] [PubMed] [Google Scholar]
- 21.Nordström L, Sembo S, Eden P, et al. SOX11 and TP53 add prognostic information to MIPI in a homogenously treated cohort of mantle cell lymphoma–a Nordic Lymphoma Group study. Br J Haematol. 2014;166(1):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]


