Fig. 8.
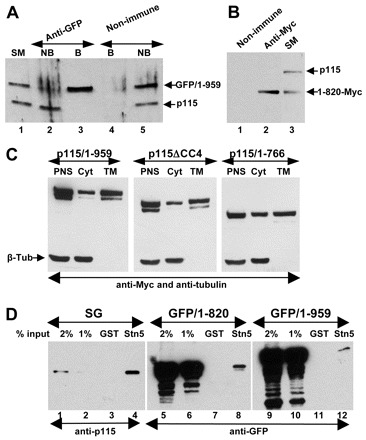
Interactions of mutant p115 with cellular proteins. (A) HeLa cells transfected with GFP-p115/1-959 for 18 hours were lysed and lysates immunoprecipitated with non-immune or anti-GFP antibodies. The starting material (SM), non-bound fractions (NB) and bound precipitates (B) were analyzed by SDS-PAGE and immunoblotted with anti-p115 antibodies. Similar levels of endogenous p115 and GFP-p115/1-959 are present in the SM (lane 1). GFP-p115/1-959 is depleted from the NB fraction (lane 2). Only GFP-p115/1-959 is recovered in the precipitate (lane 3). (B) HeLa cells transfected with Myc-p115/1-820 for 18 hours were lysed and the lysates immunoprecipitated with unspecific (lane 1) or anti-Myc (lane 2) antibodies. The starting material (lane 3) and precipitates were analyzed by SDS-PAGE and immunoblotted with anti-p115 antibodies. Similar levels of endogenous p115 and Myc-p115/1-820 are present in SM (lane 3). Only Myc-p115/1-820 is recovered in the precipitate (lane 2). (C) HeLa cells transfected with Myc-tagged p115/1-959, p115ΔCC4 or p115/1-766 for 18 hours were fractionated to generate a post-nuclear supernatant (PNS) which was subsequently fractionated into cytosol (Cyt) and total membranes (TM). Similar levels of p115/1-959, p115ΔCC4 and p115/1-766 associate with membranes. (D) GST or GST with the cytoplasmic domain of syntaxin-5 were incubated with solubilized stacked Golgi (SG) fraction (lanes 1–4), lysate from HeLa cells transfected with GFP-p115/1-820 (lanes 5–8) or with GFP-p115/1-959 (lanes 9–12). Bound proteins were eluted and analyzed by SDS-PAGE and immunoblotting with anti-p115 antibodies. The starting material for each binding is shown (lanes 1–2, 5–6, and 9–10). Full-length p115/1-959 and p115/1-820 bind to beads containing syntaxin-5 (lanes 4, 8 and 12).
