Abstract
Background
Videoscopic left cardiac sympathetic denervation (LCSD) is an adjunct therapy for reduction of arrhythmia-induced events in patients with long-QT syndrome (LQTS). LCSD reduces LQTS-triggered breakthrough cardiac events. The temporal effects of QTc changes post-LCSD have not been studied.
Methods
We utilized continuous QTc monitoring on 72 patients with LQTS. We evaluated acute and long-term QTc changes in comparison to 12-lead ECG-derived QTc values prior to surgery, 24 hours post-surgery, and at follow up ≥3 months.
Results
72 patients underwent LCSD at our institution (46% male, mean age at LCSD was 14 ± 10 years). The mean baseline, pre-LCSD QTc was 505 ± 56 ms, which had decreased significantly at ≥3 months post-LCSD to 491 ± 40 ms (p = 0.001). QTc monitoring revealed that the majority of the cohort (53/72; 74%) had a transient increase >30 ms in QTc from baseline, with an average maximum increase of 72 ± 30 ms. Resolution within 10 ms of baseline or less occurred in 57% (30/53) at 24 hours post-LCSD.
Conclusions
Although LQTS patients may have a paradoxically increased QTc post-LCSD, the effects are transient in most patients. Importantly, no patients experienced any arrhythmias in the postoperative setting related to this transient rise in QTc.
Keywords: QTc, arrhythmia, syncope, sudden cardiac death, ICD, Clinical: Electrophysiology – long QT syndrome, Clinical: Non-invasive techniques – electrocardiography, Left cardiac sympathetic denervation, 12609
Introduction
Congenital long-QT syndrome (LQTS) predisposes patients to a higher risk of sudden cardiac death (SCD) from lethal ventricular dysrhythmias originating from QT prolongation and torsades de pointes 1. The substrate for arrhythmogenesis involves mutations in cardiac ion channels comprising the cardiac action potential 1 contributing to abnormal cardiac repolarization, and an increased time of ventricular repolarization 2,3. Currently, treatment options for LQTS patients involve the use of beta-blockers 4, placement of an implantable cardioverter-defibrillator (ICD) 5, and for certain patients, left cardiac sympathetic denervation (LCSD) 6-9.
LCSD involves surgical resection of the lower half of the stellate ganglion and sympathetic chain from T1 to T4 6, 7. LCSD's anti-fibrillatory effect is thought to be due to an alteration in cardiac repolarization based on a reduction in sympathetic activation and dispersion of repolarization at the ventricular level 3, 7. Previously, Schwartz et al. have shown that in high-risk individuals, the QTc shortens by a mean of 39 ms at 6 months following LCSD 10. However, the temporal nature of this alteration in repolarization remains undefined, especially in the acute phase occurring post denervation. We therefore utilized a continuous, real-time QT monitoring analysis software system to record QTc data post-operatively in order to assess acute changes in QTc as well as short- and long-term follow-up in QTc post LCSD.
Methods
Cohort definition
We retrospectively analyzed a cohort comprised of both clinically or genetically characterized LQTS patients at Mayo Clinic in Rochester, MN who had undergone videoscopic LCSD between November 2005 and May 2012 (n = 82 patients) at our institution. Of these, 72 patients had continuous QT/QTc interval monitoring post-procedurally with the Phillips IntelliVue MP70R/Phillips DXL 12 lead software system. QTc values were determined from standard 12-lead ECG data performed pre-LCSD, and at 24 hours and 3 months post-surgery with Bazett's equation. A detailed review of the electronic medical record was performed to ascertain baseline characteristics such as demographics, results of LQTS genetic testing, surgical indication, risk level, β-blocker use, and evaluation of ICD therapies. Changes in QTc (either short-term or long-term) were correlated to outcome and specifically a breakthrough cardiac event, which were defined as a syncopal episode, seizure, aborted cardiac arrest, or appropriate VF-terminating ICD shock. These were noted via communication to or with a single provider at the time of follow-up in the Long QT clinic.
LCSD procedure
The method for surgical denervation was performed as previously described6, 11. In brief, the left sympathetic ganglia were exposed and isolated using a video-assisted transthoracic surgical approach. Sympathectomy involved the lower half of the stellate ganglion, T1, as well as T2 through T4 levels of the sympathetic chain through division of the major rami communicans and branches traveling to the left ventricle. After procedural completion, patients were monitored closely in an intensive care setting for continuous cardiac monitoring 6, 11.
QTc monitoring
QTc values were obtained from standard 12-lead ECGs performed 1-2 days pre-LCSD, 24 hours post-LCSD, and at least 3 months post-LCSD. The continuous QTc monitoring in the postoperative period was obtained using continuous, beat-to-beat QTc detection monitoring with the IntelliVue MP70/Phillips DXL 12-lead software system as previously described and validated12, 13. We used time of skin closure after the LCSD procedure as the start time of monitoring QTc post-denervation in order to maintain standardization for the temporal ECG profile. We extracted QTc data points at 2-hour intervals post-procedure from the electronic patient records to compare and evaluate the temporal QTc profiles post-LCSD for all patients in order to standardize timing of QTc data point acquisition amongst our cohort.
Statistical methods
All data are reported as mean and standard deviation of the mean unless otherwise mentioned. SAS (Cary, NC) was utilized for statistical analysis. Paired t-tests were used to compare QTc values between time points. Two-sample t-tests were used to compare these mean values between groups. The Chi-square test for independence was used to test for differences in categorical variables between groups. A two-tailed p-value of <0.05 was considered threshold for statistical significance for all tests.
Results
I. LQTS cohort demographics
Overall, 72 patients with an LQTS genotype/phenotype that had post-surgical continuous QTc monitoring data were included in this study. There was a slight female predominance (40/72, 56%) in this cohort (Table 1). The cohort involved mostly young adolescents, with an average age at diagnosis of 11 ± 10 years. The average age at the time of LCSD procedure was 14 ± 10 years. The average duration of the surgical procedure was 42 ± 14 minutes. All patient demographics are summarized in Table 1.
Table 1.
Baseline Demographics of Cohort
| Sex (male/female) | 32/40 |
| Age at diagnosis (yr) | 11±10 |
| Age at LCSD (yr) | 14±10 |
| Baseline QTc (ms) | 505±56 |
| ICD pre-LCSD, no. (%) | 18 (28%) |
| B-blocker, no. (%) | 67 (93%) |
| Genotype positive, no. (%) | 67 (93%) |
| LQT1 | 36 (50%) |
| LQT2 | 17 (24%) |
| LQT3 | 5 (7%) |
| JLNS | 6 (8%) |
| Other | 3 (4%) |
| Genotype negative/Phenotype positive | 4 (6%) |
| Symptom at diagnosis, no. (%) | 38 (53%) |
| None | 34 (47%) |
| Syncope | 26 (36%) |
| Cardiac arrest | 7 (10%) |
| Fetal arrhythmia | 5 (7%) |
| Indication for LCSD, no. (%) | |
| Beta-blocker intolerance | 28 (39%) |
| Additional protection | 19 (26%) |
| Breakthrough cardiac events | 15 (21%) |
| Severe LQTS/high-risk | 10 (14%) |
Genotyping was performed on this cohort during their clinical evaluation. Almost all of these patients were genotype-positive (67/72; 93%). The majority of patients were either type 1 LQTS (LQT1; 36/72; 50%) or type 2 LQTS (LQT2; 17/72; 24%), comprising about three-quarters of the composition of the cohort. Six patients had LQT1 with concomitant deafness, also known as Jervell- and Lange-Nielsen Syndrome (6/72; 8%). The remainder of the cohort was composed of patients that were either LQT3 (5/72; 7%), genotype-negative/phenotype positive (4/72; 6%), or had >1 LQTS-associated mutation (3/72; 4%). In 5 patients, genotype data was unknown or the patient had elected not to undergo genetic testing.
About half of the patients were symptomatic with previous LQTS-triggered cardiac events at the time of diagnosis (38/72; 53%). The other patients were asymptomatic (34/72; 47%) at presentation. Among symptomatic patients, the most common event was syncope (26/72; 36%); others presented with either fetal cardiac arrhythmias (5/72; 7%) or aborted cardiac arrest (7/72; 10%). Among the asymptomatic patients, the most common reason for LCSD was that the patient required additional protection from arrhythmogenic events (28/72; 39%), had beta-blocker intolerance (19/72; 26%), or was categorized as having severe LQTS (7/72; 10%).
Twenty-five percent (18/72) of patients had an ICD in place prior to LCSD, while almost all patients were on β-blocker therapy prior to surgery (67/72; 93%). The majority of patients with an indication for LCSD were because of β-blocker intolerance (28/72; 39%) or for additional protection from arrhythmia-induced events (19/72; 26%). The remainder of the cohort had LCSD performed because of breakthrough cardiac events despite optimal medical therapy (15/72; 21%), or were deemed to have high risk/severe LQTS and required additional protection for potentially life-threatening LQTS-associated arrhythmias (10/72; 14%).
II. Temporal changes in the QTc post-LCSD
The average baseline QTc pre-LCSD for the study cohort was 505 ± 56 ms. There was no statistically significant difference in mean QTc values 24 hours post-LCSD (502 ± 60 ms; p = 0.48) compared to the pre-LCSD QTc. However, for the 57 patients with ECGs available for review ≥3 months post-LCSD, a statistical reduction in QTc to 491 ± 40 ms (p = 0.001) was seen (average time to follow-up ECG was 1.3 ± 0.8 years).
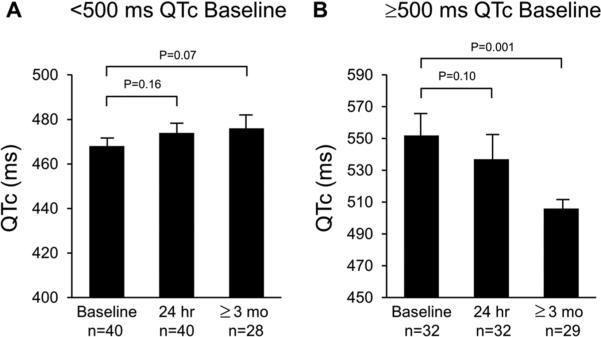
To evaluate the effect on QTc for the patients with extreme QT prolongation, we subsequently performed a subset analysis for patients with or without a pre-LCSD QTc>500ms (Figure 1). Among patients with a baseline QTc <500 ms, the average baseline QTc was 468 ± 21 ms (n = 40) and no significant differences in the QTc were noted at 24 hours post-denervation (n=40; mean QTc 474 ± 27 ms; p=0. 16) or at ≥3 months follow-up (n=28; mean QTc 476 ± 27 ms; p=0.07). In contrast, among patients with a baseline QTc≥500 ms (n=32), a mean QTc of 552 ± 51 ms was observed at baseline and did not significantly differ from their mean QTc 24 hours post-denervation (537 ± 70 ms (p= 0.1). However, among the 29 patients with baseline QTc>500 ms who had follow-up ECG at ≥3 months post-LCSD, mean QTc was significantly lower than baseline (QTc 506 ± 47 ms; p<0.001) (Figure 1).
Figure 1. Temporal changes in QTc post-LCSD are more pronounced in patients with more prolonged QTc.
Bar graphs representing comparisons of QTc values of sub-cohorts at baseline, 24hr, and ≥3 month values in sub-cohorts <500 ms (panel A) and ≥ 500 ms (panel B). T-test for comparison amongst means was used to calculate p values, which are shown above bars reflecting comparisons.
III. Dynamic changes in QTc in the first 24 hours post-LCSD
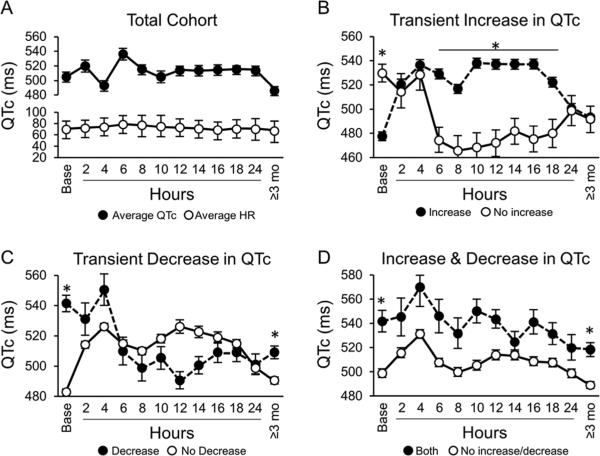
Utilizing continuous, beat-to-beat QTc monitoring post-LCSD, significant fluctuations in QTc occurred during the postoperative period as well as when compared to pre-operative values in our overall cohort. We then stratified the QTc changes according to a magnitude of >30 ms change from baseline to identify sub-cohorts with either a decrease, increase, or both increase/decrease in QTc>30 ms during the 24-hour monitoring period post-surgery. Figure 2 illustrates dynamicity of QTc values over time from baseline values, 24-hour post LCSD monitoring, and ≥3 months follow-up in our total cohort (Figure 2a), as well as in sub-cohorts with an increase (Figure 2b- filled circle curve), decrease (Figure 2c- filled circle curve), or both increase and decrease (Figure 2d – filled circle curve) in QTc>30 ms in the acute setting post LCSD. Most notably from these curves, there is a statistically significant difference (Figure 2b) in acute QTc changes at 8 hours, and this remains sustained through the 18-hour time point during post LCSD monitoring.
Figure 2. Dynamic changes in QTc occurring over first 24 hours post-LCSD.
Temporal values of QTc values showing dynamicity over time. Average values at each time point are shown along with standard error of the mean unless otherwise mentioned.
Panel A. Overall cohort changes in average QTc and average HR following LCSD (±SD).
Panel B: Comparison of QTc fluctuations post LCSD in subgroup with transient increase in QTc > 30 ms from baseline (filled circles) versus the rest of the cohort without such an increase (open circles).
Panel C: Comparison of QTc fluctuations post LCSD in subgroup with transient decrease in QTc >30 ms from (filled circles) versus the rest of the cohort without such a decrease (open circles).
Panel D: Comparison of QTc fluctuations post LCSD in subgroup with both a transient increase and decrease in QTc >30 ms from baseline (filled circles) versus the rest of the cohort without such an increase/decrease (open circles).
* Asterix above mean value denotes a statistically significant difference between the means at a given time point (rank sum analysis at each time point was used); p value < 0.05 was considered statistically significant.
Overall, the majority of the patients (53/72; 74%) had a transient increase of >30 ms in QTc from baseline at some point during the monitoring period. The average maximum increase in these patients found was 72 ± 30 ms. Importantly, although the transient increase could be substantially raised from baseline and in a large number of patients increased above the pro-arrhythmic threshold of 500 ms, none of these patients had a documented arrhythmia or LQTS associated event during the postoperative period. In patients with a transient increase in QTc, it normalized to within 10 ms of baseline levels or less in 57% of patients (30/53). In 70% of patients (30/43), QTc resolved at ≥3 months follow-up ECG. Additionally, in just over one-third of the cohort (27/72; 38%) a decrease of >30 ms during the monitoring period compared to baseline was observed (Fig 2b). A small proportion of our cohort (12/72; 17%) had both an increase as well as a decrease of 30 ms in QTc from baseline (Fig 2c). Similar to patients with an increase in QTc, none of the patients experienced a LQTS-associated breakthrough cardiac event during the 24-hour postoperative period.
IV. Dynamic changes in QTc do not identify a subset of patients without breakthrough cardiac events post-LCSD
Overall, 24% of the patients (17/72) experienced ≥1 breakthrough cardiac event post-LCSD during a follow-up period of 2.2 ± 1.7 years. Evaluating QTc variability observed by continuous QT monitoring, there was no statistically significant difference in the percentage of patients with breakthrough cardiac events based upon their acute QTc response. Furthermore, even though 14 LQTS patients had both an acute and sustained decrease (>3 months follow-up) in QTc >30 ms after LCSD, this did not correlate with the absence of an event as 43% of patients (6/14) had at least one event following LCSD. Therefore, although there is dynamicity to the QTc interval in the post-LCSD monitoring period, the acute and short-term effects do not predict which patients are likely to be most protected by LCSD.
Discussion
Short- and long-term outcomes of LCSD for various cardiac arrhythmogenic diseases have been well documented 6, 8, 10, 14-16. However, to the best of our knowledge, this is the first systematic and large-scale study of LQTS patients post-LCSD evaluating the dynamic QTc fluctuations within the 24 hours post-surgical period using continuous QTc monitoring. In our study cohort, consisting of patients with severe LQTS and baseline QTc of 505 ms, almost all were on β-blocker therapy, and about half of this cohort was symptomatic at presentation. The major results of this study are therefore best understood in their clinical context.
Significant decrease in QTc post-LCSD surgery
Almost 20 years ago, Ouriel and Moss published on a small cohort of 10 patients with LQTS (mean baseline QTc 540 ± 10 ms (range 460-600 ms) and showed QTc had decreased 30 ± 10 ms post LCSD 15. In these patients, the mean QTc was 490 ± 10 ms (440-540 range) at the time of hospital discharge. This apparent QTc attenuating effect with LCSD was replicated by Schwartz and colleagues in 2004 10. It has been suggested that the benefit from sympathetic denervation in LQTS is based on a reduction in the “trigger” via reduced norepinephrine activity at the local ventricular tissue level, and a change in “substrate” for arrhythmogenesis through a shortened time for ventricular repolarization 7, 10. In a pivotal study led by Schwartz et al in high risk LQTS patients undergoing LCSD, the average QTc pre-procedure was 543 ± 65 ms in 130 patients 10. Pre- and post-procedure comparison data was available for 85 patients and showed a shortening in the QTc interval on average of 39 ± 54 ms; p<0.001 10.
In comparison to our study, our cohort had a much lower average QTc value and overall we did not see the same amount of QTc attenuation. However, after stratifying our cohort according to a baseline QTc of 500 ms or greater to include higher risk QT patients, the magnitude of QTc attenuation was indeed comparable as we had an average reduction of 46 ms.
Work from the Schwartz group has also suggested that further decreases in QTc were correlated with more patients being asymptomatic post LCSD 16. These authors also pointed out the important fact that the efficacy of LCSD should not be based simply on QTc normalization alone, but rather on a decrease in arrhythmia-related syncopal events 16; a sentiment echoed and demonstrated by several papers on outcomes of LCSD surgery 10, 14, 16. While these findings are similar, none of these studies were able to evaluate the acute and continuous QTc changes immediately following LCSD and correlate these to long-term outcome and success of the denervation surgery.
Paradoxical increase in QTc post-LCSD
The perioperative management of patients with LQTS is complicated by the fact that these patients are at a comparatively higher risk for Torsades de Pointes (TdP) than the general population 17. Multiple factors including QT-prolonging pharmacologic medications, postoperative electrolyte disturbances, and catecholamine surges may further put these patients at risk to ventricular arrhythmias in the setting of surgery. Interestingly, in approximately 75% of patients in our cohort a significant, a paradoxical rise of >30ms in QTc post-LCSD procedure was observed. In a postoperative setting (most commonly the ICU), these patients had a notable maximum increase in QTc interval of 72 ± 30 ms on continuous QTc monitoring. Importantly, although the transient increase in QTc was substantially higher than their already prolonged baseline and for many patients crossed the pro-arrhythmic risk marker of 500ms, none of these patients had an arrhythmic event during the postoperative period. Even more reassuring was the finding that the QTc returned back to baseline in about half of these patients by 24 hours post-procedure, and an even higher percentage was decreased back to baseline or lower at follow-up of at least 3 months. These data provide reassurance for those caring for such patients in the postoperative period, including the patients and family members watching the monitors, when this marked postoperative spike in the QTc is observed. Specifically, this acute QTc behavior should now be anticipated without elevation of concern for an impending arrhythmia.
Potential Explanation and/or Mechanisms of Acute QTc Alterations
The QTc monitoring was done using the Phillips IntelliVue system and utilizing a beat-to- beat account of calculated QTc according to Bazett's formula. Although acute changes in QT were not associated with QT-related events during their hospitalization and were not associated with the absence of breakthrough AEs post-LCSD, our data underscore the fact that continuous QTc monitoring still has significant value for LQTS patient's post-LCSD. This is particularly underscored as approximately three-quarters of our cohort had an increase of at least 30 ms above baseline QTc and an average increase of 75 ms. Clearly, these data strongly argue for close monitoring of these patients, especially when they may potentially be at a QTc value well over 500 ms and at great risk of developing LQT-associated TdP. In fact, in the hospital setting, this could be compounded by drug-induced TdP if medications from the QT drug list are required for postoperative recovery. Continuous QTc monitoring can also provide a timely trajectory of the QTc in the postoperative setting, and this increased vigilance is necessary for patient safety, especially when prescribing medications that might aggravate the QT interval. However, in all these situations, it must be recognized and the staff must be reminded to “treat the patient, not the monitor”. While the QTc might increase significantly, if the patient is asymptomatic and conscious, no additional treatment is required. Further, the patient, family and staff should be reassured that in most cases, the QTc will decrease over time.
Nagele et al. reported that post-op QTc prolongation was common 30 minutes post operatively and likely due to medications given during surgery or surgical stress 18. This study was done on approximately 500 adult patients undergoing non-cardiac surgery; one of these patients had an increase in QTc from 439 to 468 ms and developed TdP 18. As a result, in our denervation program, we try to avoid as much as possible agents that will stimulate sympathetic tone. Instead, midazolam is used for sedation/anxiolysis and isoflurane/sevoflurane or propofol for induction and maintenance 19.
It is also possible differences in pain and pain sensation in the postoperative setting may lead to a differential effect on QTc based on type, timing, and dose of medications given. Additionally, this may also have been the case for patients who took different amounts of anxiolytics, anti-emetics, and other QT prolonging agents in the postoperative period 19. Though speculative, it is most likely that the acute fluctuation in QTc is due to an imbalance of parasympathetic and sympathetic tone post-denervation, and we performed continuous monitoring, for the first time, thereby exposing this period of re-equilibration of the cardiac autonomic system.
Implications for Clinical Practice and Future Study
Although these data provide initial reassurance of escalating QTc values in LQTS patients undergoing LCSD, this message must be taken in the proper clinical context. Although this is the first report of this phenomenon, this is in a relatively small cohort of patients, and therefore our group and others will need to continue to accumulate more data, with longer follow-up to better define the natural history of this QTc dynamicity and its prognostic efforts. Furthermore, we wish to attract attention to the critical need for a multi-disciplinary approach to the post-op care of these patients. At our center, we follow a strict protocol with avoidance of QT prolonging medications and triggers19. In light of this QTc continuous monitoring data, adherence to measures and education for caregivers is of critical importance to the safety of these patients.
Limitations
This was a retrospective analysis and is limited by its inherent bias to such a study. We did not have complete post LCSD ECGs at least 3 months post-LCSD for all patients; and as a large tertiary referral center, we cannot be completely sure all post denervation outcomes were complete such as breakthrough cardiac events, unless patients returned for follow-up or results were made available to our clinic. Surgically, although the denervation is visualized at the T1-T4 level, we cannot comment on the efficacy of the procedure from a microscopic view, or from variations inherent to individual patient's innervation patterns at the ventricle. Furthermore, we cannot make any definitive claims regarding the effect changes in autonomic tone, medication, and pain levels for each individual patient at the time of QTc calculation. This underscores the need for prospective evaluation that takes into account these factors so that a correlation can be systematically evaluated.
Conclusions
In our large retrospective cohort study in LQTS patients with LCSD performed at a single institution, we found that dramatic QTc alteration commonly occurred in the immediate postoperative period. Even though some patients’ QTc was immediately prolonged, most of these resolved within 24-hour post-procedure and the majority had resolved by 3 months follow-up. Although the transient attenuation of QTc was predictive of QTc attenuation at 3 months follow-up, these effects did not identify a sub-cohort of patients without any breakthrough cardiac events. Further study involving longer term follow-up in patients, as well as additional patients involved in the cohort will provide information on whether or not these acute QTc change can be predictive of protective response to ICD shocks or breakthrough events in the follow-up period. Furthermore, since these were resting ECG values, the real benefit post-LCSD in these patients may be brought out with exercise testing to evaluate the susceptibility of the heart in a higher catecholamine state and increased heart rate.
Footnotes
Dr. Asirvatham receives no significant honoraria and/or consulting for Abiomed, AtriCure, Biotronik, Boston Scientific, Medtronic, Spectranetics, St. Jude, Sanofi-Aventis, Wolters Kluwer, and Elsevier. Dr. Ackerman receives royalties from Transgenomic (Familion), and is a consultant for Boston Scientific, Gilead Sciences, Medtronic, and St. Jude Medical. Other authors: No disclosures.
References
- 1.Giudicessi JR, Ackerman MJ. Potassium-channel mutations and cardiac arrhythmias--diagnosis and therapy. Nat Rev Cardiol. 2012;9:319–332. doi: 10.1038/nrcardio.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathias A, Moss AJ, Lopes CM, Barsheshet A, McNitt S, Zareba W, Robinson JL, Locati EH, Ackerman MJ, Benhorin J, Kaufman ES, Platonov PG, Qi M, Shimizu W, Towbin JA, Michael Vincent G, Wilde AAM, Zhang L, Goldenberg I. Prognostic implications of mutation-specific QTc standard deviation in congenital long QT syndrome. Heart Rhythm. 2013;10:720–725. doi: 10.1016/j.hrthm.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Diehl L, Schwartz PJ. Dispersion of the QT interval. A marker of therapeutic efficacy in the idiopathic long QT syndrome. Circulation. 1994;89:1681–1689. doi: 10.1161/01.cir.89.4.1681. [DOI] [PubMed] [Google Scholar]
- 4.Chockalingam P, Crotti L, Girardengo G, Johnson JN, Harris KM, van der Heijden JF, Hauer RNW, Beckmann BM, Spazzolini C, Rordorf R, Rydberg A, Clur S-AB, Fischer M, van den Heuvel F, Kääb S, Blom NA, Ackerman MJ, Schwartz PJ, Wilde AAM. Not All Beta-Blockers Are Equal in the Management of Long QT Syndrome Types 1 and 2: Higher Recurrence of Events Under Metoprolol. Journal of the American College of Cardiology. 2012;60:2092–2099. doi: 10.1016/j.jacc.2012.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horner JM, Kinoshita M, Webster TL, Haglund CM, Friedman PA, Ackerman MJ. Implantable cardioverter defibrillator therapy for congenital long QT syndrome: A single-center experience. Heart Rhythm. 2010;7:1616–1622. doi: 10.1016/j.hrthm.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–759. doi: 10.1016/j.hrthm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. European Heart Journal. 2013;34:3109–3116. doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Hu D, Shang L, Ma S, Liu W, Li Y, Ma Z, Tang C, Mei Y, Wang L. Surgical left cardiac sympathetic denervation for long QT syndrome: effects on QT interval and heart rate. Heart Vessels. 2005:137–141. doi: 10.1007/s00380-005-0820-1. [DOI] [PubMed] [Google Scholar]
- 9.Atallah J, Fynn-Thompson F, Cecchin F, DiBardino DJ, Walsh EP, Berul CI. Video-Assisted Thoracoscopic Cardiac Denervation: A Potential Novel Therapeutic Option for Children With Intractable Ventricular Arrhythmias. The Annals of Thoracic Surgery. 2008;86:1620–1625. doi: 10.1016/j.athoracsur.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, Bloise R, De Ferrari GM, Klersy C, Moss AJ, Zareba W, Robinson JL, Hall WJ, Brink PA, Toivonen L, Epstein AE, Li C, Hu D. Left Cardiac Sympathetic Denervation in the Management of High-Risk Patients Affected by the Long-QT Syndrome. Circulation. 2004;109:1826–1833. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 11.Coleman MA, Bos JM, Johnson JN, Owen HJ, Deschamps C, Moir C, Ackerman MJ. Videoscopic Left Cardiac Sympathetic Denervation for Patients With Recurrent Ventricular Fibrillation/Malignant Ventricular Arrhythmia Syndromes Besides Congenital Long-QT Syndrome. Circulation: Arrhythmia and Electrophysiology. 2012;5:782–788. doi: 10.1161/CIRCEP.112.971754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou SH, Helfenbein ED, Lindauer JM, Gregg RE, Feild DQ. Philips QT Interval Measurement Algorithms for Diagnostic, Ambulatory, and Patient Monitoring ECG Applications. Annals of Noninvasive Electrocardiology. 2009;14:S3–S8. doi: 10.1111/j.1542-474X.2008.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helfenbein ED, Ackerman MJ, Rautaharju PM, Zhou SH, Gregg RE, Lindauer JM, Miller D, Wang JJ, Kresge SS, Babaeizadeh S, Feild DQ, Michaud FP. An algorithm for QT interval monitoring in neonatal intensive care units. Journal of Electrocardiology. 2007;40:S103–S110. doi: 10.1016/j.jelectrocard.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Bos JM, Bos KM, Johnson JN, Moir C, Ackerman MJ. Left Cardiac Sympathetic Denervation in Long QT Syndrome: Analysis of Therapeutic Nonresponders. Circulation: Arrhythmia and Electrophysiology. 2013;6:705–711. doi: 10.1161/CIRCEP.113.000102. [DOI] [PubMed] [Google Scholar]
- 15.Ouriel K, Moss AJ. Long QT syndrome: an indication for cervicothoracic sympathectomy. Cardiovascular Surgery. 1995;3:475–478. doi: 10.1016/0967-2109(95)94444-2. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome. A worldwide report. Circulation. 1991;84:503–511. doi: 10.1161/01.cir.84.2.503. [DOI] [PubMed] [Google Scholar]
- 17.Kies SJ, Pabelick CM, Hurley HA, White RD, Ackerman MJ. Anesthesia for Patients with Congenital Long QT Syndrome. Anesthesiology. 2005;102:204–210. doi: 10.1097/00000542-200501000-00029. [DOI] [PubMed] [Google Scholar]
- 18.Nagele P, Pal S, Brown F, Blood J, Miller J, Johnson J. Postoperative QT-interval prolongation in patients undergoing non-cardiac surgery under general anesthesia. Anesthesiology. 2012;117:321–328. doi: 10.1097/ALN.0b013e31825e6eb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenyon CA, Flick R, Moir C, Ackerman MJ, Pabelick CM. Anesthesia for videoscopic left cardiac sympathetic denervation in children with congenital long QT syndrome and catecholaminergic polymorphic ventricular tachycardia – a case series. Pediatric Anesthesia. 2010;20:465–470. doi: 10.1111/j.1460-9592.2010.03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]