Article first published online 23 March 2015.
Key Words: inflammatory bowel disease, atherosclerosis, arterial stiffness, endothelial dysfunction
Abstract
Background:
Patients with inflammatory bowel disease (IBD) have increased risk for atherosclerosis. The cause of increased cardiovascular risk is not fully understood. Chronic inflammatory process may predispose to atherosclerosis. We aimed primarily to investigate subclinical atherosclerosis in patients with IBD, by measuring carotid femoral pulse wave velocity (PWV), carotid intima media thickness, and flow-mediated dilatation compared with matched normal controls.
Methods:
Patients with IBD were recruited among individuals proven to have Crohn's disease (CD) and ulcerative colitis (UC). To exclude any interference of confounding factors for endothelial dysfunction or atherosclerosis, we studied a specifically selected group with no additional cardiovascular risk. PWV, carotid intima media thickness, and flow-mediated dilatation levels were measured in all patients and controls.
Results:
The carotid femoral PWV levels were 8.13 ± 1.61 m/sec in the patients with UC, 8.16 ± 1.74 m/sec in the patients with CD, and 6.85 ± 0.95 m/sec in the healthy subjects. The levels of carotid femoral PWV were significantly higher in patients with CD and UC than control group (P < 0.001). Flow-mediated dilatation levels were significantly decreased in patients with UC and CD (9.6% ± 5.1% versus 108% ± 4.4%) when compared with control subjects (15.1% ± 9.7%) (P = 0.004). No significant difference in carotid intima media thickness was detected between UC, CD, and control groups (P = 0.152).
Conclusions:
This study suggests that patients with IBD without traditional cardiovascular risk factors have increased risk of endothelial dysfunction and atherosclerosis.
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and ulcerative colitis (UC), is an autoimmune disorder with unknown etiology, which is characterized by chronic relapsing–remitting inflammation of the gastrointestinal tract.1 Patients with IBD have lower traditional cardiovascular risk factors than the general population. However, some studies suggested that patients with IBD have increased risk for cardiovascular events and atherosclerosis.2 The cause of increased cardiovascular risk is not fully understood. Chronic inflammatory process may predispose to atherosclerosis. In fact, the effect of chronic inflammation on development of atherosclerosis has already been demonstrated in various chronic inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus.3,4 Inflammation contributes to all stages of atherosclerosis, from initiation of atherosclerosis to eventual plaque rupture and thrombosis.5
Chronic and relapsing inflammation is an important feature of IBD. Proinflammatory cytokines, such as C-reactive protein (CRP), tumor necrosis factor alpha, and interleukin, are typically elevated in course of IBD and have been shown to be associated with subclinical atherosclerosis.6–8 Moreover, in the healthy population, CRP is a strong independent risk factor for the cardiovascular disease (CVD).9 These inflammatory cytokines causes endothelial dysfunction and structural vessel abnormalities in the arterial wall as a result of the changes in lipid levels, oxidative stress, and insulin resistance.10,11
Recent studies have demonstrated that subclinical atherosclerosis in patients with IBD is increased due to chronic inflammation, metabolic changes, chronic steroid treatment, vitamin deficiencies, and hypercoagulability.12,13 There are a few noninvasive procedures, such as carotid intima media thickness (CIMT) measurement and flow-mediated dilatation (FMD) to detect subclinical atherosclerosis. Arterial stiffness (AS) is another new noninvasive method, which has been used recently. Elevated AS is indicator of structural and functional changes in vessel wall.14 Carotid femoral pulse wave velocity (cf-PWV) has been accepted as the gold standard for evaluation of AS and is a strong predictor of future cardiovascular events and all-cause mortality.15,16
To date, only a few studies have examined relationship between AS and subclinical atherosclerosis in patients with IBD.17–20 In this study, we aimed primarily to investigate subclinical atherosclerosis in patients with IBD, by measuring cf-PWV, CIMT, and FMD compared with matched normal controls. Our secondary aim was to demonstrate the relationship between these parameters and disease variables (disease duration and extent). To exclude any interference of confounding factors for endothelial dysfunction or atherosclerosis, we studied a specifically selected group with no additional cardiovascular risk factors, such as hypertension (HT), diabetes mellitus (DM), renal failure, and hyperlipidemia.
METHODS
Study Population
Our study was cross-sectional and observational in design. Patients with IBD were recruited among 162 individuals proven to have CD and UC with clinical, radiological, endoscopic, and histopathological findings, who attended the clinic of the Gastroenterology Department, Gulhane School of Medicine, Ankara, Turkey, between September 2013 and September 2014. A detailed history, including smoking status (current smoker and nonsmoker), disease duration and extent, medications, and previously intestinal surgery was taken from each participant. The extent of UC and CD was determined by Montreal classification (proctitis, left-sided colitis, and pancolitis for UC and terminal ileitis, colitis, and ileocolitis for CD).21 Patients with previous CVD, DM, HT, chronic renal failure, infectious, and inflammatory disorders other than CD and UC were excluded. Control subjects matched for age and gender were recruited from healthy volunteers without risk factors of atherosclerosis. The study was approved by the local ethics commitee of Gulhane School of Medicine, in accordance with declaration of Helsinki and written informed consent obtained from all patients and healthy subjects before entering the study.
Clinical and Laboratory Assessments
Body weight and height were measured in subjects with light clothes and without shoes. Body mass index was calculated as the ratio of weight (in kilograms)/height2 (in square meter). Blood pressure was measured 3 times after a 5-minute rest in sitting position, and the average of 3 measurements was considered as the level of blood pressure. HT was defined as documentation of blood pressure higher than 140/90 mm Hg or use of antihypertensive agents. DM was defined as the presence of fasting plasma glucose level over 126 mg/dL or glucose level over 200 mg/dL at any time of measurement or the use of antidiabetic drugs. Dyslipidemia was defined as the presence of total cholesterol level ≥260 mg/dL or low-density lipoprotein (LDL) level ≥160 mg/dL or the use of lipid-lowering agents. All laboratory tests were studied from venous blood after a 12-hour fasting. High-sensitive CRP was measured by using a nephelometric method. Hemoglobin, platelet, white blood cell, serum levels of fasting plasma glucose, uric acid, high-density lipoprotein, triglycerides, and creatinine were determined by using autoanalyzers. LDL was calculated by using the Friedewald's formula.22
PWV Measurements
PWV, a measure of AS, was calculated using an automatic arteriograph device (TensioMed Ltd., Budapest, Hungary). Measurements in both IBD and controls were made after resting for 5 minutes in a supine position and in a reserved quiet room. A single experienced internal medicine specialist who was blind to the clinical characteristics of the participants took the measurements. Participants did not consume any food or drink and did not smoke for at least 30 minutes leading up to the measurement period. The distance between the jugular notch and the symphysis pubis of each individual was measured, and the data were recorded on the device. During the measurement period, brachial artery occlusion was made, and the blood flow was ceased as a part of the process.
CIMT Measurements
Measurements of intima media thickness (IMT) were performed on all patients using B-mode ultrasonography (Acuson S3000; Siemens, Germany) by using a high-resolution, 18-MHz linear array transducer. For each subject, measurements were carried out while the subject was in a supine position and had the neck rotated to the opposite side of examination. A single trained radiologist who was blind to the participants' clinical characteristics took the measurements in a dark quiet room. The region of interest for the measurement of IMT of both the right and the left common carotid artery was selected 1 cm proximal to the common carotid artery bifurcation. Longitudinal static images were analyzed using semiautomated software (Syngo Arterial Health Package). The transducer was manually placed on a 1-cm segment of the region of interest, and the IMT was then automatically measured by calculating the distance between the lumen–intima and the media–adventitia interfaces in the far wall of the region of interest. If the reader decided that the measurement was not adequate, the CIMT was again measured using the same method. The CIMT of both the left and right common carotid artery was measured only once, and the CIMT value was calculated by averaging measurements of the left and right common carotid IMT.
FMD Measurements
The right brachial artery was imaged with a 18-MHz linear array transducer on M-mode (Acuson S3000; Siemens). The same single trained radiologist studied the subjects in a controlled environment while they were in the supine position after completing a minimum of 4-hour fast. A cuff was placed at the upper arm approximately 4 cm above the antecubital crease. The first baseline arterial diameter was measured. A 3-minute period of forearm blood flow occlusion is provided by using a cuff above 200 mm Hg. After cuff deflation and post–blood flow occlusion, the arterial diameter was measured. FMD at 1 minute postischemia (i.e., 100 × [Diameter (1 min) − Diameter(basal)/Diameter(basal)]) was used to represent spontaneous endothelial function. Significantly endothelial dysfunction was defined as FMD <10%.
Statistical Analyses
SPSS (Statistical Package for the Social Sciences, version 17.0; SPSS Inc., Chicago, IL) computer program was used for all statistical calculations. Results are reported as the mean ± SD, frequency, and percent. The Kolmogorov–Smirnov test was used to determine the distribution characteristics of continuous variables. The normally distributed variables were compared with one-way analysis of variance for multiple-group comparison and a Tukey's test was used for post hoc. The Kruskal–Wallis test was used in a multiple-group comparison of variables without normal distribution, and a Bonferroni-adjusted Mann–Whitney U test was used as a post hoc. Categorical variables were compared by chi-square or Fisher's exact test. Pearson's correlation analysis was used to evaluate the relationship between continuous variables. A multivariate analysis of variance was used to assess the effects of age and other clinical factors on vascular parameters. Statistical significance was defined as P < 0.05.
RESULTS
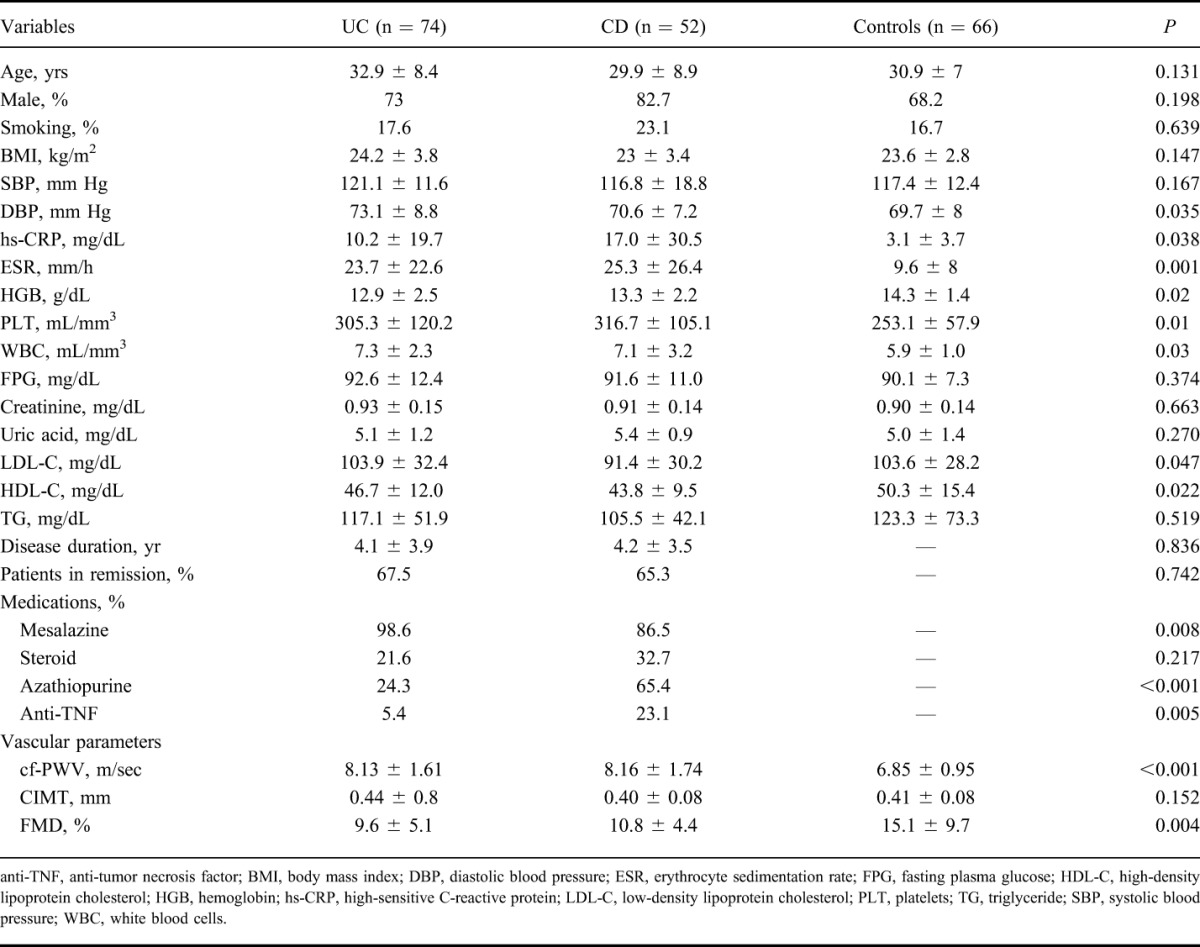
A total of 192 cases, consisting of 74 patients with UC, 52 patients with CD, and 66 healthy control subjects, were included in the study. The baseline characteristics of the study population are summarized in Table 1. No significant differences existed among UC, CD, and the control group regarding demographic features (P > 0.05), whereas inflammatory markers, such as erythrocyte sedimentation rate, high-sensitive CRP, white blood cell, and platelet, were significantly higher in patients with UC and CD than in the control group (P < 0.05). Moreover, no significant difference was found between UC and CD in terms of these inflammatory parameters (P > 0.05). The diastolic blood pressure levels were higher in UC patients than in the controls (P < 0.05). Hemoglobin levels were lower in UC than in the controls (P < 0.05), and high-density lipoprotein C levels were higher in the healthy subjects than in the patients with CD (P < 0.05).
TABLE 1.
The Baseline Characteristics of Study Population
Of the patients with UC, 10.8% (n = 8) had proctitis, 56.7% (n = 42) had left-sided colitis, and 32.4% (n = 24) had pancolitis. Of the patients with CD, 46.1% (n = 24) had ileum, 13.4% (n = 7) had colon, and 40.4% (n = 21) had ileocolonic involvement. Two patients had upper gastrointestinal involvement in CD; 1 patient had only duodenal involvement and 1 patient had both upper gastrointestinal and ileocolonic involvement. These patients were excluded from the study due to statistical weaknesses. Anti-tumor necrosis factor drugs, such as infliximab and adalimumab, were administered in 12 patients with CD and 4 patients with UC (P = 0.005). In addition, 9 (17.3%) patients with CD were operated on due to complications of CD, such as stricture, fistula, and abscess, but no patient with UC had intestinal surgery. In patients with UC, 1 patient (1.3%) had cerebrovascular involvement, as extraintestinal manifestation and colon adenocarcinoma was observed in 1 patient (1.3%). In patients with CD, no extraintestinal manifestation and malignancy were detected. Moreover, 67% of UC and 65% of CD were in remission due to anti-inflammatory drugs during measurements.
The cf-PWV levels were 8.13 ± 1.61 m/sec in the patients with UC, 8.16 ± 1.74 m/sec in the patients with CD, and 6.85 ± 0.95 m/sec in the healthy subjects. The levels of cf-PWV were significantly higher in patients with CD and UC than in the control groups (P < 0.001), but no significant difference was found between UC and CD (P > 0.05). We found significantly decreased FMD levels in patients with UC and CD (9.6% ± 5.1% versus 10.8% ± 4.4%) compared with control subjects (15.1% ± 9.7%) (P < 0.05), but FMD levels did not differ significantly between patients with UC and CD (P > 0.05). However, no difference in CIMT was detected between UC, CD, and the controls (Table 1).
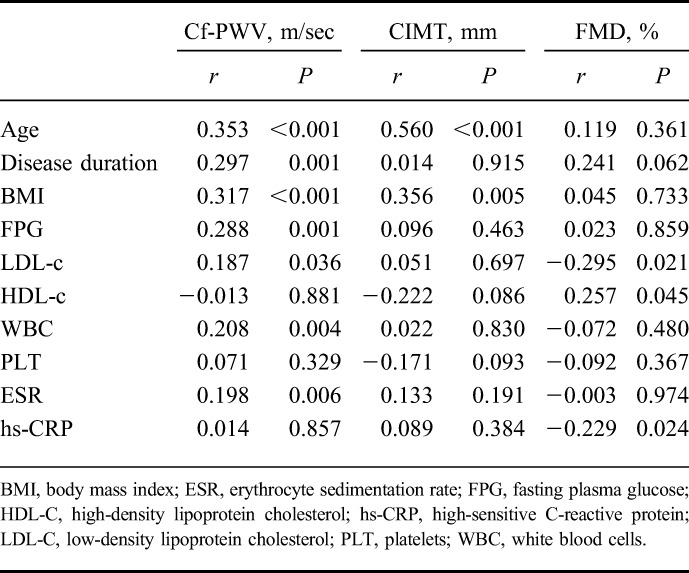
We also assessed the relationship between vascular parameters (cf-PWV, FMD, and CIMT) and disease variables (duration and extent). We did not find any significant relationships between disease extent, cf-PWV, FMD, and CIMT (P > 0.05) (Table 2). The mean disease duration was 4.1 ± 3.9 years in UC and 4.2 ± 3.5 years in CD. A significant correlation was found between cf-PWV and disease duration (P = 0.001, r = 0.297), but CIMT and FMD levels were not correlated with disease duration. In addition, cf-PWV levels were correlated positively with age, body mass index, white blood cell, erythrocyte sedimentation rate, fasting plasma glucose, and LDL-c levels. Multivariate analyses confirmed the correlation between disease duration and cf-PWV with age independently (β = 0.202, P < 0.05). Furthermore, FMD levels were correlated negatively with high-sensitive CRP and LDL-c and positively with high-density lipoprotein-c. A significant correlation was found between CIMT levels, age, and body mass index (Table 3).
TABLE 2.
The Values of Cf-PWV, CIMT, and FMD in Groups by Extent of IBD
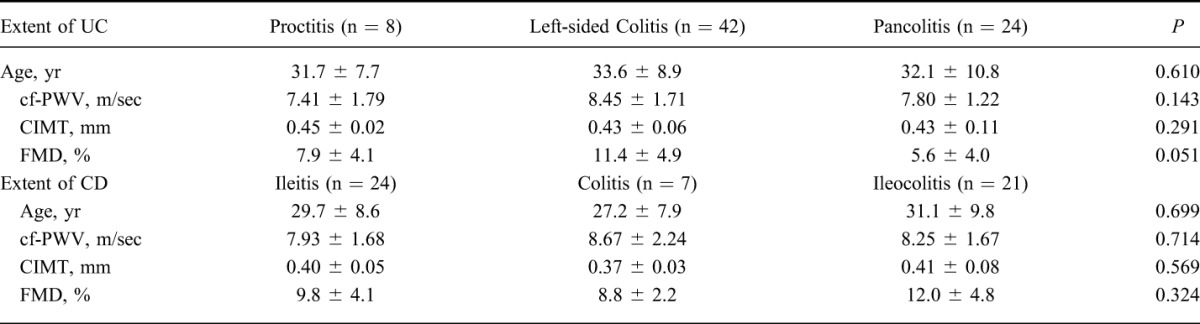
TABLE 3.
Correlation Analyses of Cf-PWV, CIMT and FMD Levels with Disease Duration and Various Parameters
In a subgroup analysis, we have evaluated the effect of medications on vascular parameters in patients with IBD. Patients with IBD were divided into 3 groups; mesalazine (n = 69), mesalazine + azathiopurine (n = 38), and anti-tumor necrosis factor (n = 16). The remaining 3 patients had used only azathiopurine. These patients were not included in the analysis due to small sample size. No difference existed between the groups in terms of cf-PWV, CIMT, and FMD (P = 0.326, P = 0.305, and P = 0.702, respectively) (Fig. 1).
FIGURE 1.
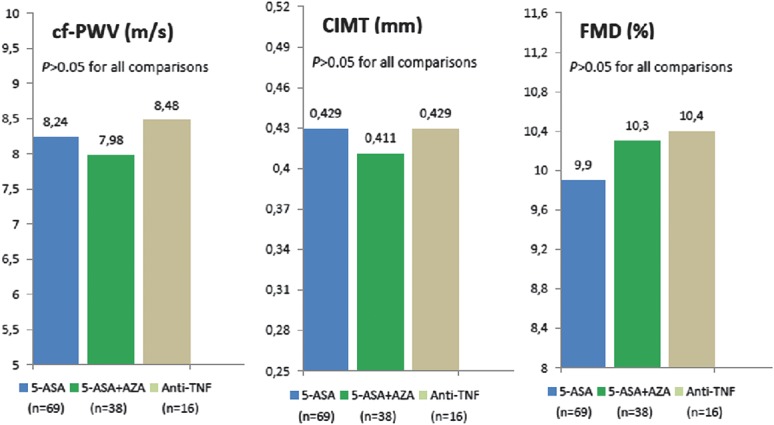
Medications received and vascular parameters. 5-ASA, mesalazine; AZA, azathiopurine; TNF, tumor necrosis factor.
DISCUSSION
In this cross-sectional study, we have demonstrated that AS is increased in patients with IBD, who have no cardiovascular risk factors. Although FMD was impaired in patients with UC and CD when compared with healthy controls, no differences in CIMT levels were found between UC, CD, and healthy subjects. In addition, there was positive correlation between cf-PWV and disease duration. To the best of our knowledge, this study has the most comprehensive data for evaluating the relationship between atherosclerosis, UC, and CD.
AS has been known as an independent predictor of all-cause and cardiovascular mortality in patients with cardiovascular risk factors.23,24 The measurement of cf-PWV is a simple, reproducible, relatively easily, and noninvasive method for evaluating CVD in the general population. Increased AS in inflammatory diseases, such as SLE and RA, has been reported to be dependent on inflammation, but only a few studies involving patients with IBD have investigated the relationship between IBD and AS. Akdoğan et al17 reported that PWV and CIMT increased and correlated with disease involvement and disease activity in 37 patients with UC. Zanoli et al demonstrated that AS was significantly higher in patients with IBD compared with healthy participants and can be reversed by immunomodulatory therapy.18,19 In another study, Korkmaz et al20 showed that PWV, homocysteine levels, insulin, and insulin resistance were increased in patients with IBD, and that homocysteine levels, insulin, and insulin resistance levels were correlated positively with PWV values. In this study, we found that cf-PWV increased in patients with UC and CD when compared with control group, and no difference was found between UC and CD. When the above-mentioned studies were analyzed separately, it can be seen that some of the patients with IBD had metabolic confounders, such as HT and DM.17 In addition, mean age of individuals in these studies was higher than this study.17,19,20 Despite these confounding risk factors, our findings are consistent with previous studies in terms of increased AS. All of these findings suggest that IBD may be an independent risk factor for increased AS. Moreover, when taken together with results of previous studies,18–20 disease duration could be an important predictor of impaired AS, independently of age.
Endothelium plays an important role in the maintenance of vascular function and structure through the production and release of nitric oxide under the influence of agonists and through shear stress.25 Endothelial function in clinical research is usually used to evaluate early atherosclerosis, measuring the degree of vasodilation in patients who have cardiovascular risk factors. Endothelial dysfunction is one of the earliest indicators of atherosclerosis and is measured by the brachial artery FMD. FMD, a well-established noninvasive measure of endothelial function, was reported by 4 clinical studies in patients with IBD so far. Schinzari et al26 reported that endothelial function was impaired in CD but not in UC. Principi et al27 evaluated endothelial dysfunction in 26 patients with CD and 23 with UC and showed that FMD was significantly lower in both CD and UC than in the control groups. Kocaman et al28 revealed that endothelial dysfunction is associated with UC. Moreover, it was significantly correlated with disease activity. In another study, Kayahan et al13 found that both endothelium-dependent and endothelium-independent vasodilatation were impaired in patients with IBD, and also, sCD40L levels were negatively associated with endothelial function. In our study, we demonstrated that endothelial function was impaired in patients with CD and UC when compared with healthy subjects, but there was no difference between CD and UC in terms of endothelial dysfunction. Thus, in light of literature and our results, we suggest that patients with IBD have an elevated risk of endothelial dysfunction.
IMT reflects early structural changes in the arterial wall.29 Measuring the IMT of the carotid artery through ultrasonography has been to independently predict future cardiovascular events in asymptomatic individuals.30 To date, CIMT in patients with IBD has been investigated to evaluate early atherosclerosis in many studies. Of 9 studies, 5 reported increased CIMT in patients with IBD compared with controls,17,31–34 and 4 studies found no difference between IBD and controls.13,27,35,36 Thus, these results showed that the available data regarding the association of IBD and CIMT still remains controversial, and that additional information is required. In this study, we did not find any difference regarding CIMT levels among subjects UC, CD, and healthy controls. We suggest some possible explanations for the lack of a relationship between IBD and CIMT. A potential explanation for this remarkable finding, cf-PWV, and FMD represent an early marker of endothelial dysfunction and functional disorders in the arterial vessel wall, whereas CIMT reflects structural alterations in intima and media layers. Atherosclerosis is a complex process initiated by endothelial dysfunction, which is related to stiffness of the arterial wall caused by the proliferation of vascular smooth muscle cells and an increased production of extracellular macromolecules, such as fibrillar collagen. This step is followed by structural alterations in the arterial vessel wall and atherosclerotic plaque formation leading to CVD.37 Therefore, when CIMT is increased, endothelial function has already been impaired in the arterial vessel wall. However, even if endothelial function has been impaired, the IMT level could be still within a normal range. Thus, we think that the duration of the disease was too short to have an effect on CIMT. In our study, the mean disease duration was 4.5 years and lower than the above-mentioned studies.33,34 A longer exposure time to chronic inflammation may be required to develop increased CIMT. Another explanation could be higher mean ages of the participants and the cardiovascular risk factors of some patients, such as DM and HT, in previous studies.17,31,32,34 It is well known that a strong and independent relationship exists among age, cardiovascular risk factors, and CIMT.38
Because IBD is characterized by episodes of remission and exacerbation, disease activity may not provide sufficient data regarding the potential impact of chronic inflammation on atherosclerosis in the course of IBD. Many previous studies did not demonstrate a significant association between disease activity and atherosclerosis.20,32,34–36 For this reason, we think that disease duration and extent are more reliable markers of inflammation exposure. Moreover, as the disease duration and extent is increased, the risk of atherosclerosis development may increase independently of age. In this study, we found that the disease duration was significantly correlated with cf-PWV but not with CIMT and FMD. To explain this discrepancy, we suggest that PWV reflects a different entity of vascular damage than FMD or CIMT in young adults. In older adults, they present similar adverse vascular wall damage due to more advanced atherosclerosis.39 Moreover, Lunder et al40 reported that no correlation existed between PWV, FMD, and CIMT in healthy middle-aged man. Furthermore, we did not observe any effect of IBD extent and medications on vascular parameters. In previous studies, although Akdogan et al17 reported the correlation between disease extent and vascular parameters (AS and CIMT), Theocharidou et al34 did not found any relationship between disease extent and CIMT. These results suggested that exposure time to cardiovascular risk factors and inflammation is a more important predictor of atherosclerosis in patients with IBD.
Several limitations to this study should be noted. First, it has a cross-sectional nature that prevents any definitive causal inference. Second, there was small sample size to evaluate the relationship between vascular parameters and disease extent. The proctitis and colitis subgroup had quite small participants due to strict inclusion criteria. Third, inflammation was successfully suppressed with anti-inflammatory therapy in our patients. Hence, the majority of patients with IBD were in remission, and the number of patients with complicated IBD was smaller. Finally, some participants in the study population were smokers. Smoking is an important risk factor for CVD, but it does not cause accelerated atherosclerosis, such as DM and HT. To prevent interference of this confounding factor, control subjects were matched for smoking with patients with IBD.
In conclusion, this study suggests that patients with IBD, without traditional cardiovascular risk factors have an increased risk of endothelial dysfunction and atherosclerosis. Structural changes in the arterial vessel wall occur because of long-term exposure to inflammation or cardiovascular risk factors after endothelial dysfunction. In addition, we concluded that inflammation exposure time is a more effective factor than disease activity or extent in the development of atherosclerosis.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. [DOI] [PubMed] [Google Scholar]
- 2.Yarur AJ, Deshpande AR, Pechman DM, et al. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741–747. [DOI] [PubMed] [Google Scholar]
- 3.Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. [DOI] [PubMed] [Google Scholar]
- 4.Salmon JE, Roman MJ. Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am J Med. 2008;121:S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Athyros VG, Kakafika AI, Karagiannis A, et al. Do we need to consider inflammatory markers when we treat atherosclerotic disease? Atherosclerosis. 2008;200:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel disease. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. [DOI] [PubMed] [Google Scholar]
- 8.Rho YH, Chung CP, Oeser A, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61:1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Buring JE, Shih J, et al. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;25:731–733. [DOI] [PubMed] [Google Scholar]
- 10.Chung CP, Oeser A, Solus JF, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008;58:2105–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz O, Oberhauser F, Saech J, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5:e14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theocharidou E, Gossios TD, Giouleme O, et al. Carotid intima-media thickness in patients with inflammatory bowel disease: a systematic review. Angiology. 2014;65:284–293. [DOI] [PubMed] [Google Scholar]
- 13.Kayahan H, Sari I, Cullu N, et al. Evaluation of early atherosclerosis in patients with inflammatory bowel disease. Dig Dis Sci. 2012;57:2137–2143. [DOI] [PubMed] [Google Scholar]
- 14.Otsuka K, Fukuda S, Shimada K, et al. Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res. 2014;37:1014–1020. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 16.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 17.Akdoğan RA, Durakoğlugil ME, Kocaman SA, et al. Increased pulse wave velocity and carotid intima-media thickness in patients with ulcerative colitis. Dig Dis Sci. 2013;58:2293–2300. [DOI] [PubMed] [Google Scholar]
- 18.Zanoli L, Cannavò M, Rastelli S, et al. Arterial stiffness is increased in patients with inflammatory bowel disease. J Hypertens. 2012;30:1775–1781. [DOI] [PubMed] [Google Scholar]
- 19.Zanoli L, Rastelli S, Inserra G, et al. Increased arterial stiffness in inflammatory bowel diseases is dependent upon inflammation and reduced by immunomodulatory drugs. Atherosclerosis. 2014;234:346–351. [DOI] [PubMed] [Google Scholar]
- 20.Korkmaz H, Sahin F, Ipekci SH, et al. Increased pulse wave velocity and relationship with inflammation, insulin, and insulin resistance in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2014;26:725–732. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 22.Fridewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–230. [DOI] [PubMed] [Google Scholar]
- 24.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 25.Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997;20:3–10. [PubMed] [Google Scholar]
- 26.Schinzari F, Armuzzi A, De Pascalis B, et al. Tumor necrosis factor-alpha antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin Pharmacol Ther. 2008;83:70–76. [DOI] [PubMed] [Google Scholar]
- 27.Principi M, Mastrolonardo M, Scicchitano P, et al. Endothelial function and cardiovascular risk in active inflammatory bowel diseases. J Crohns Colitis. 2013;7:e427–e433. [DOI] [PubMed] [Google Scholar]
- 28.Kocaman O, Sahin T, Aygun C, et al. Endothelial dysfunction in patients with ulcerative colitis. Inflamm Bowel Dis. 2006;12:166–171. [DOI] [PubMed] [Google Scholar]
- 29.Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;18:346–349. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary DH, Polak JF, Kronmal RA, et al. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 31.Dagli N, Poyrazoglu OK, Dagli AF, et al. Is inflammatory bowel disease a risk factor for early atherosclerosis? Angiology. 2010;61:198–204. [DOI] [PubMed] [Google Scholar]
- 32.van Leuven SI, Hezemans R, Levels JH, et al. Enhanced atherogenesis and altered high density lipoprotein in patients with Crohn's disease. J Lipid Res. 2007;48:2640–2646. [DOI] [PubMed] [Google Scholar]
- 33.Papa A, Santoliquido A, Danese S, et al. Increased carotid intima-media thickness in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005;22:839–846. [DOI] [PubMed] [Google Scholar]
- 34.Theocharidou E, Gossios TD, Griva T, et al. Is there an association between inflammatory bowel diseases and carotid intima-media thickness? preliminary data. Angiology. 2013;65:543–550. [DOI] [PubMed] [Google Scholar]
- 35.Broide E, Schopan A, Zaretsky M, et al. Intima-media thickness of the common carotid artery is not significantly higher in Crohn's disease patients compared to healthy population. Dig Dis Sci. 2011;56:197–202. [DOI] [PubMed] [Google Scholar]
- 36.Maharshak N, Arbel Y, Bornstein NM, et al. Inflammatory bowel disease is not associated with increased intimal media thickening. Am J Gastroenterol. 2007;102:1050–1055. [DOI] [PubMed] [Google Scholar]
- 37.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 39.Koivistoinen T, Virtanen M, Hutri-Kähönen N, et al. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: the Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis. 2012;220:387–393. [DOI] [PubMed] [Google Scholar]
- 40.Lunder M, Janic M, Kejzar N, et al. Associations among different functional and structural arterial wall properties and their relations to traditional cardiovascular risk factors in healthy subjects: a cross-sectional study. BMC Cardiovasc Disord. 2012;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]


