Figure 2.
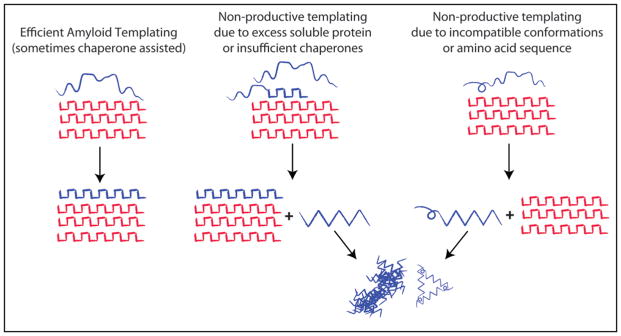
Amyloid and Non-productive templating. Amyloid fibrils grow by causing proteins of the same amino-acid sequence to adopt the same amyloid conformation. This is referred to as amyloid templating and involves the efficient addition of monomers or oligomeric species to the amyloid fiber, which maybe assisted by specific chaperones (e.g., Rnq1 and the Hsp40 Sis1, Sup35NM and Hsp104,49). If the amount of substrate exceeds the cellular capacity for amyloid conversion or if amino acid sequences are incompatible, the interaction of substrate with the amyloid fibrils may give rise to other abnormal conformational species, which may go on to form toxic oligomers and amorphous aggregates (Rnq1, PrP and Het-s/S). We refer to this as non-productive templating.
