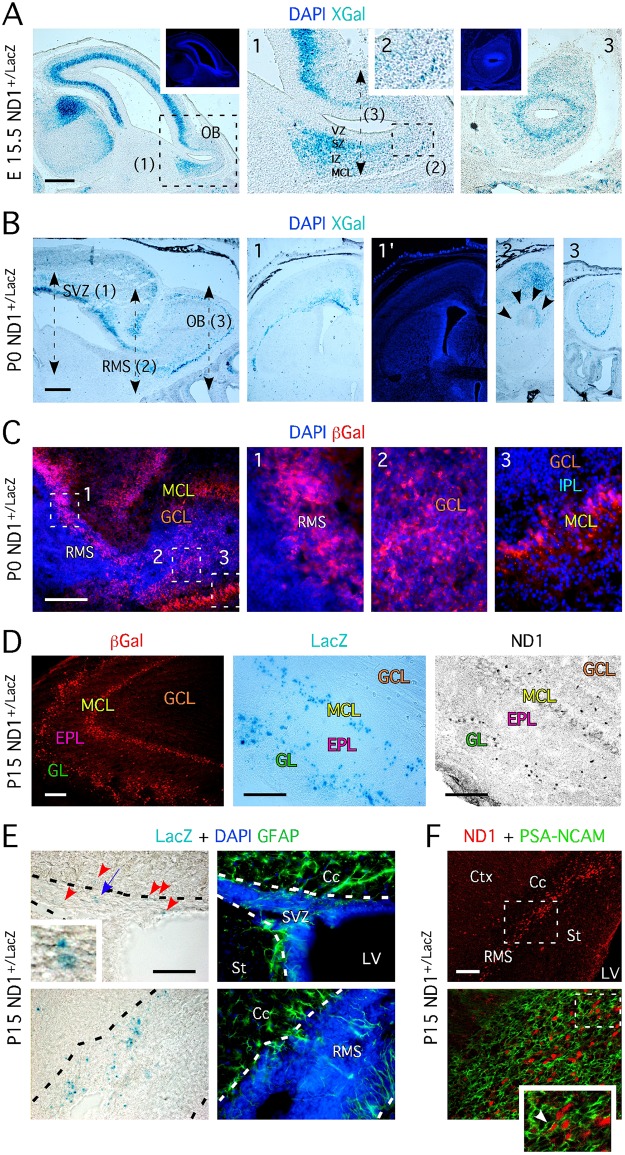
Fig 1. ND1 defines distinct populations of OB neurons born during the development and after birth.
A- XGal staining on sagittal section of ND1+/LacZ embryos aged E15.5 reveals the presence of β-Gal+ cells in the SZ, IZ and MCL of the forming OB. Insets show higher magnification of regions indicated by dashed frame. Panel 3 represents XGal staining performed on coronal section at the level of the black dashed double arrowed line. Insets located in the upper part show DAPI staining (in blue). B- XGal staining on sagittal section of ND1+/LacZ newborn mice aged P0 shows the presence of β-Gal+ cells in the SVZ, the RMS, and the MCL of the OB. Insets (1, 2 and 3) show XGal staining on coronal sections at the level of each of these regions. DAPI staining of 1 is represented in 1’. In 2, multiple black arrowheads pinpoint at the outer and dorsal part of the RMS. C- Immunohistochemistry for β-Gal performed on sagittal section of ND1+/LacZ mice aged P0 confirms the existence of ND1+/LacZ cells revealed by XGal staining in B. Panels 1–3 represent higher magnifications of regions of interest marked by white dashed frames in the left panel. D- Numerous ND1 cells locate in the GL and MCL in the OB at P15, as revealed by XGal, β-Gal and ND1 stainings. E- ND1+/LacZ cells locate in the SVZ and RMS of 2 week-old mice and are negative for the glial fibrillary acidic protein (GFAP). Red arrowheads pinpoint at XGal+ cells. Inset represents a higher magnification of XGal+ cells pinpointed by the blue arrowhead. Black dashed lines delineate the SVZ and RMS. F- Immunohistochemistry performed on sagittal section of 2-week old ND1+/LacZ mice reveals that ND1+ are migrating PSA-NCAM+ neuroblasts. A-to F- Pictures are representative images of stainings perform on serial sections from n = 4–6 animals. VZ = ventricular zone, SZ = subependymal zone, SVZ = subventricular zone, IZ = intermediate zone, MCL = mitral cell layer, RMS = rostral migratory stream, GCL = granule cell layer, GL = glomeruli, IPL = internal plexiform layer, Cc = corpus callosum, St = striatum, LV = lateral ventricle, Ctx = cortex, OB = olfactory bulb. Scale bars: 200 μm (A-C) and 100 μm (D-F).