Figure 1.
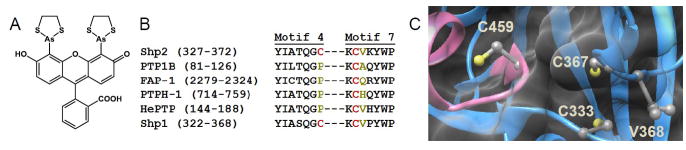
Design of classical PTP mutants that possess unnatural sensitivity to biarsenical compounds. (A) Chemical structure of the biarsenical compound FlAsH. (B) Partial amino-acid sequence alignment of the human PTPs discussed in this study, showing only structural motifs 4 and 7 of the PTP domain (as assigned by Anderson et al1). Highlighted in red are Shp2’s C333 and C367 and their cysteine counterparts in other PTPs. Highlighted in dark yellow are the amino acid residues substituted with cysteine in the present study. (The PTP-domain primary-sequence numbering varies widely due to the diversity in protein size and structure of the PTP family outside of the conserved PTP domain.42) (C) Three-dimensional structure of Shp2’s catalytic domain (PDB ID: 3B7O).30 Shp2 is shown as a blue ribbon, with the conserved active-site motif highlighted in pink and featuring the catalytic cysteine C459. The side chains of C333, C367, V368, and C459 are colored by atom type. The enzyme’s surface is rendered transparently so that the buried residues C333 and C367 can be visualized.
