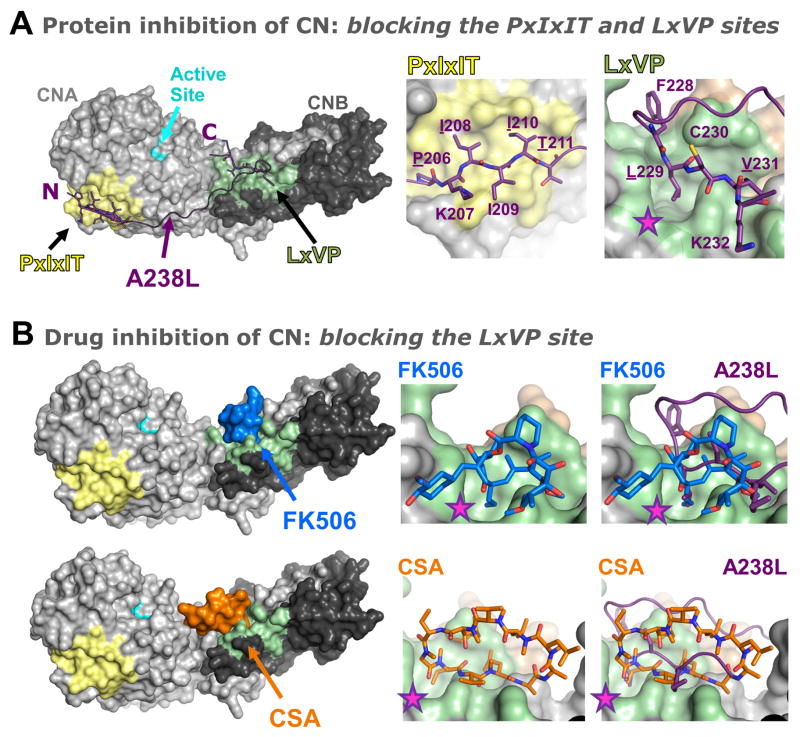
Figure 2. Inhibiting CN by blocking substrate binding.
A. The CN protein inhibitor A238L inhibits CN activity not by blocking its active site, but instead by binding to CN substrate recognition grooves, which blocks CN from binding and dephosphorylating its substrates. Left panel, surface representation of CN (light/dark grey; left panel) bound to A238L (magenta; shown as a cartoon), a potent protein inhibitor of CN from the African Swine Fever Virus. The active site is shown in light blue. A238L binds CN via both a PxIxIT sequence (middle panel) and an LxVP sequence (right panel). The deep groove in CN engaged by the ‘L’ of the LxVP motif (Leu229 in A238L) is indicated by a star (pink). B. The immunosuppressant drugs FK506 and cyclosporine A (CSA) bind directly to the CN LxVP docking groove. Left panels, surface representations of CN (light and dark grey) bound to FK506 (blue) and CSA (orange). Upper right panels, close-up views of FK506 in the CN LxVP binding groove (left) and overlay with A238L (magenta, right). The deep groove in CN engaged by the ‘L’ of the LxVP motif is indicated by a star (pink) and is fully engaged by FK506. Lower right panels, close-up views of CSA in the CN LxVP binding groove (left) and overlay with A238L (magenta, right).