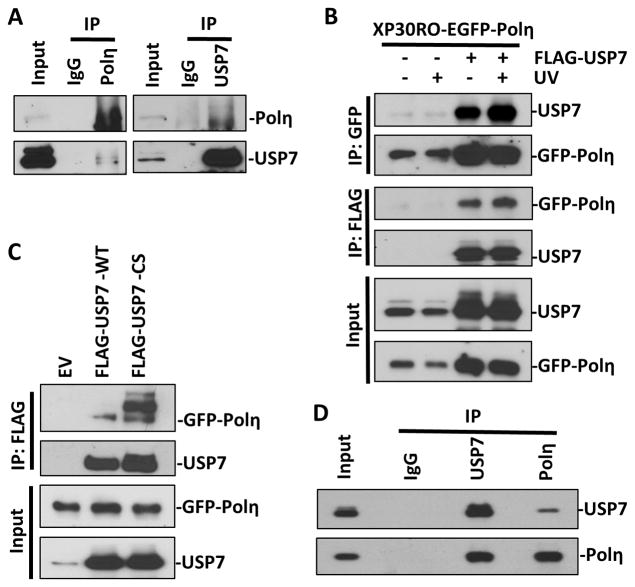
Figure 2. Polη and USP7 directly interact in vivo and in vitro.
(A) Cell lysates prepared from HCT116 cells were immunoprecipitated with anti-USP7 or anti-Polη or normal IgG antibodies and Protein G/A beads. Bound proteins were recovered and used to detect USP7 and Polη along with whole-cell lysates as input control. (B) Cell lysates, from XP30RO-EGFP-Polη cells transiently transfected with empty vector (EV) or vector encoding FLAG-tagged USP7 for 24 h and treated with or without UV (20 J/m2), were immunoprecipitated with anti-GFP or anti-FLAG beads. Bound proteins were recovered and used to detect USP7 and Polη along with whole-cell lysates as input control. (C) Cell lysates from XP30RO-EGFP-Polη cells, transiently transfected with empty vector or vectors encoding FLAG-tagged wild-type USP7 (WT) or catalytic defective (CS) for 24 h, were immunoprecipitated with anti-FLAG beads. Bound proteins were recovered and used to detect USP7 and Polη. (D) Recombinant His-tagged USP7 and Polη were mixed in in vitro binding buffer and immunoprecipitated with anti-USP7 or anti-Polη antibodies, respectively. Bound proteins were recovered and used to detect USP7 and Polη along with mixed solution as input control.