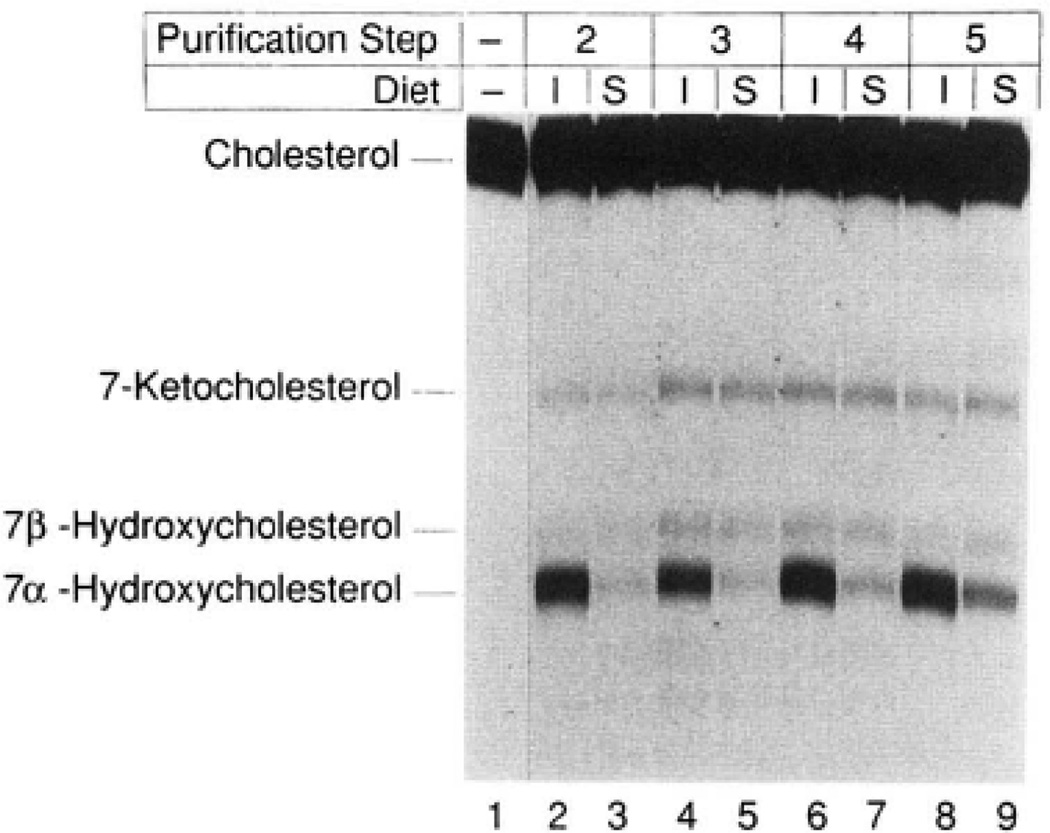
Fig. 3. Dietary regulation of 7α-hydroxylase enzyme activity.
7α-Hydroxylase was simultaneously purified from groups of 100 animals maintained for 2 weeks on rat chow supplemented with 2% cholestyramine (induced, I) or 0.2% chenodeoxycholic acid (suppressed, S) as described under “Experimental Procedures.” An equal amount of protein derived from the two groups at different purification steps was assayed for 7α-hydroxylase activity by thin layer chromatography. The purification steps correspond to those of Table I, Approximately 500 µg of protein from step 2 (lanes 2 and 3), 7 µg from step 3 (lanes 4 and 5), 6 µg from step 4 (lanes 6 and 7), and 1 µg from step 5 (lanes 8 and 9) were assayed for 5 min at 37 °C in a reaction containing 25 µM [14C] cholesterol and 1000 units of cytochrome P-450 reductase. Lane 1 contained only the starting isotope and was not subjected to solvent evaporation. The chromatogram was exposed to Kodak XAR-5 film for a period of 44 h at −70 °C. The identities of the observed sterols are indicated on the left and were determined by comparison with authentic standards. The 7-keto and 7β-hydroxylated forms of cholesterol represent spontaneous oxidation products derived from cholesterol during solvent evaporation in the workup of the various reactions.