Figure 4.
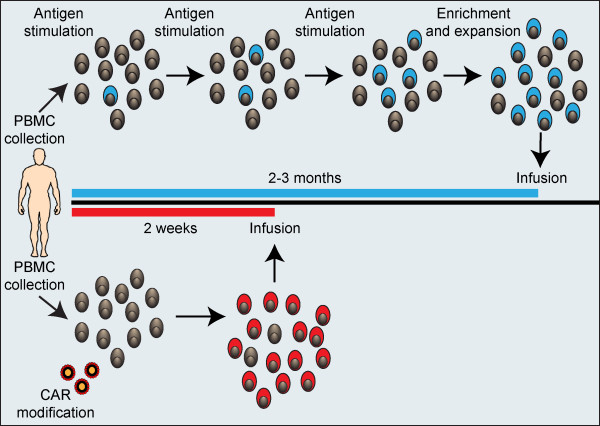
Chimeric antigen receptor modified T-lymphocyte therapy for B-cell malignancies. Generation of tumor-specific T cells by repeated antigen stimulation or genetic modification to express a tumor-targeting receptor. PBMC collected from a patient or healthy individual can be stimulated in vitro with tumor antigen at regular intervals to induce gradual enrichment of antigen-specific T cells (blue). Multiple stimulations followed by additional enrichment or expansion strategies are required to ensure sufficient antigen-specific T cells are generated. The entire process may take 2–3 months. In contrast, approaches that utilize genetic modification to redirect T cell specificity to a tumor antigen are much more rapid. PBMC can be collected from a patient or healthy donor and retrovirally or lentivirally transduced to express a tumor-reactive CAR (or TCR). The enriched CAR-modified tumor-reactive T cells (red) can be infused into the patient in as little as 1–2 weeks. Abbreviations: PBMC: Peripheral blood mononuclear cells, CAR: Chimeric antigen receptor modified T-lymphocytes. (Courtesy of The International Journal of Hematology, and Springer-Tokyo, Publisher) [102].
