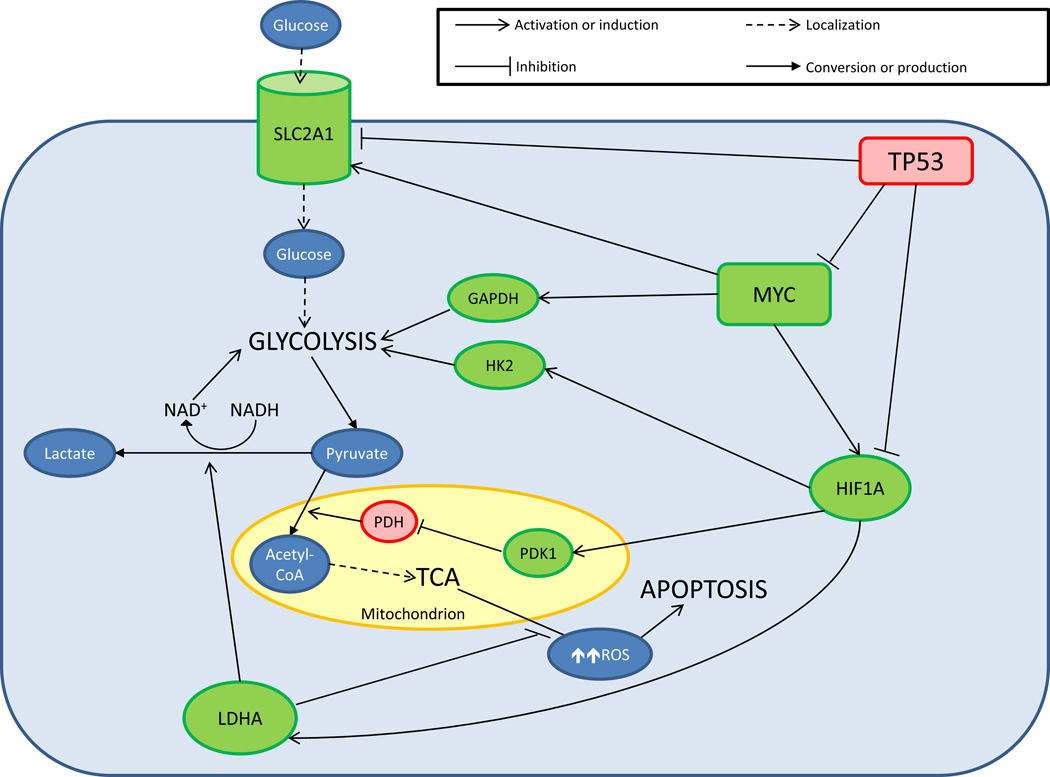
Figure 1.
Cellular energy metabolism pathways in cancer. Green molecules: upregulated or activated proteins in cancer; red molecules: downregulated or inhibited proteins in cancer. Glucose molecules enter the cell via facilitated diffusion by SLC2A1. HK2, GAPDH, and other glycolytic enzymes convert glucose to pyruvate and ATP through glycolysis. Pyruvate could either enter the mitochondria or be converted to lactate by LDHA, a process that also regenerates NAD+ required for glycolysis. Pyruvate that enters the mitochondrion is converted to acetyl-CoA by PDH prior to entering the TCA cycle. PDK1 inhibits PDH and reduces the formation of TCA cycle starting material. Increased mitochondrial energy production also generates ROS that at high levels could cause oxidative stress and subsequently apoptosis. An elevated level of LDHA is associated with reduced mitochondrial ROS production. LDHA is induced by HIF1A, which also increases the expression of PDK1 and HK2. MYC activates the expression of HIF1A, GAPDH, and SLC2A1, driving the cell to utilize aerobic glycolysis instead of mitochondrial oxidative phosphorylation. Finally, inhibition of MYC, HIF1A, and SLC2A1 by functional TP53 in a healthy cell reverses the oncogenic effects caused by overexpression of these proteins.