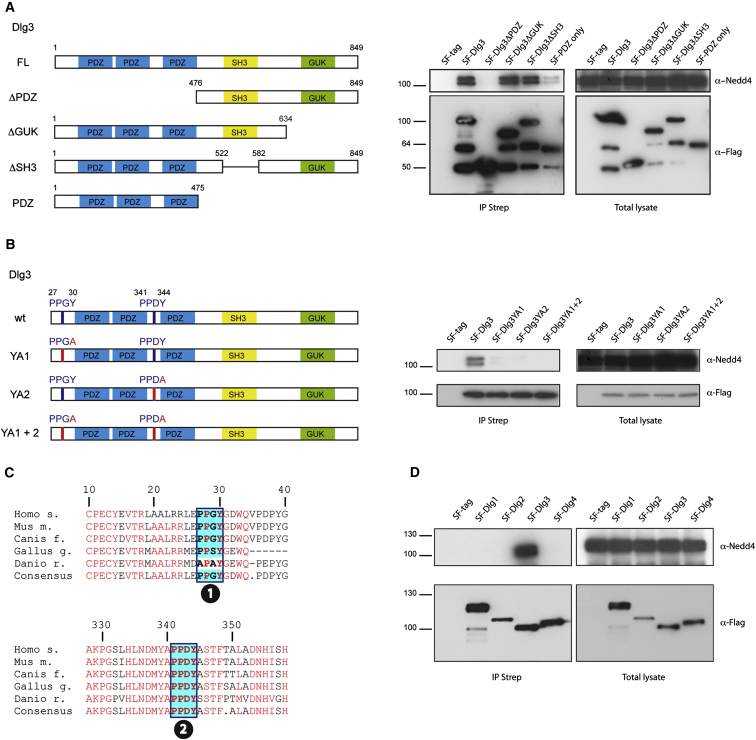
Figure 5.
Dlg3, but Not Dlg1, Dlg2, or Dlg4, Interacts with Nedd4 and Nedd4-2
(A) The PDZ domains of Dlg3 contribute to Nedd4 binding. Different deletion mutants of SF-Dlg3 (left panel) were immunoprecipitated from transiently transfected HEK293T cells via an IP Strep. The total lysate and the IP were split and subjected to anti-Nedd4 and anti-Flag via western blotting. Endogenous Nedd4 was detected in the IP Strep in the presence of full-length SF-Dlg3, SF-Dlg3ΔGUK, SF-Dlg3ΔSH3, and also weakly in the presence of SF-PDZ only. HEK293T cells expressing only the SF tag were used as a negative control. Lower bands correspond to degradation products.
(B) The Nedd4 ligases bind to Dlg3 in a PY motif-dependent manner. Indicated point mutants of Dlg3 PY motifs were generated by exchanging Tyrosine to Alanine and tagged with the SF-tag (left panel). Lysates from HEK293T cells transfected with these expression vectors were subjected to IP Strep. The lysates and IP were analyzed by western blotting using anti-Nedd4 and anti-Flag antibodies. Point mutation of both PY motifs totally abolishes interaction with the Nedd4 ligases.
(C) Multiple species ClustalW alignment of Dlg3 reveals evolutionary conservation of PY motifs (boxed regions) in vertebrates.
(D) Dlg3, but not Dlg1, Dlg2, or Dlg4, interact with the ubiquitin ligases Nedd4 and Nedd4-2. Lysates from HEK293T cells transfected with the mentioned expression vectors were subjected to IP Strep. The lysates and IP were analyzed by western blotting using anti-Nedd4 and anti-Flag antibodies. The same results were obtained using a Nedd4-2 antibody (data not shown).
See also Figure S5.