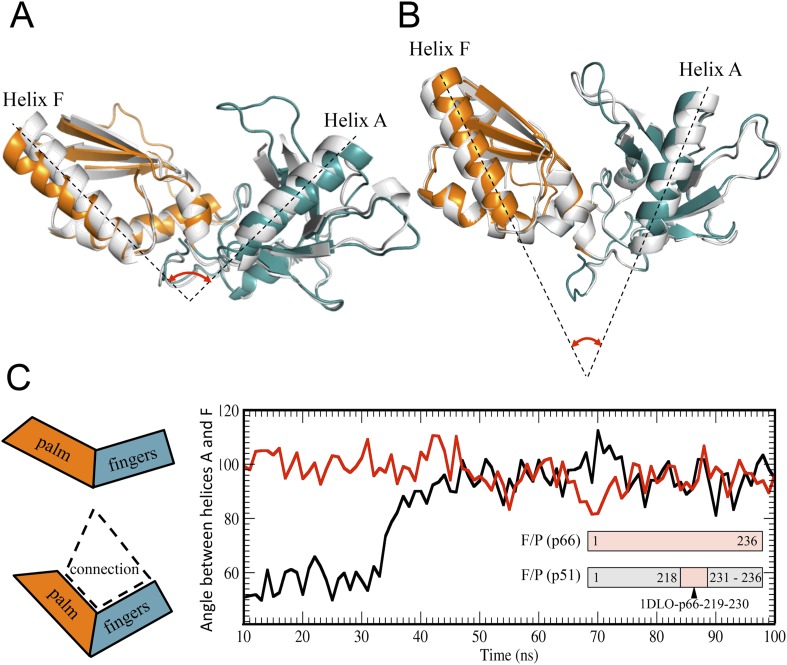
Figure 3. Alternative conformations and molecular dynamic simulations analysis of the fingers/palm subdomains.
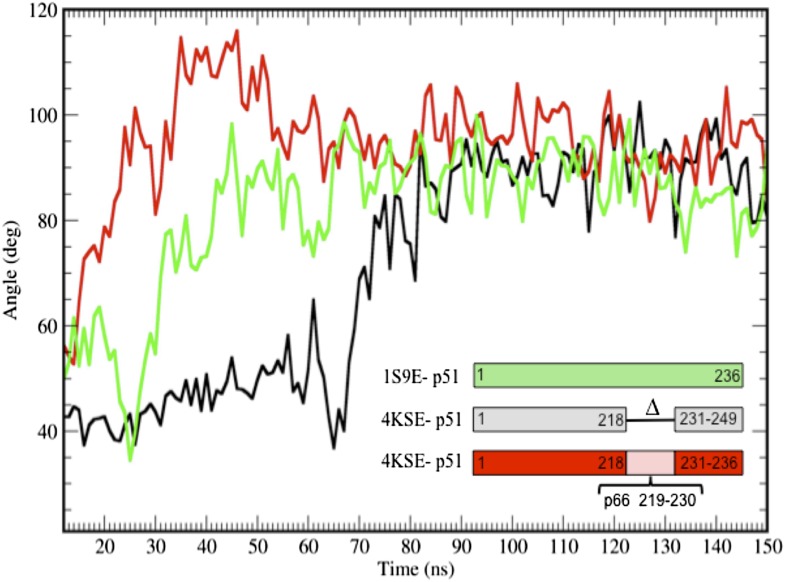
(A) Overlay of ribbon diagrams for fingers/palm residues 1–216 RT216 (pdb: 1HAR, gray) and in the p66 subunit of RT (pdb: 1DLO, fingers, teal; palm, orange). (B) Overlay of ribbon diagrams for the fingers/palm in the p51∆PL monomer (pdb: 4KSE, gray) with the corresponding region of the p51 subunit of RT (pdb: 1DLO, fingers, teal; palm, orange). The fingers/palm angle defined by helices A and F is indicated, illustrating the more acute values for the monomer and the p51 subunit, compared with an isolated fingers/palm construct and the p66 subunit. (C) Time-dependent molecular dynamics simulations of the behavior of the αAF angle for the fingers/palm starting with the p66 conformation (red) or with the p51 conformation (black). The simulations utilized residues 1–236 in the p66 and p51 subunits of RT (pdb: 1DLO) after removing all other domains at t = 0, and the missing palm loop residues in the p51 starting structure were introduced as indicated in ‘Materials and methods’. Residues included in the simulations are defined in the inset. The cartoons on the left illustrate the starting fingers/palm conformations and the proposed role of the fingers/palm:connection interface in constraining the initial αAF angle in the monomer and p51 structures.