Fig. 2.
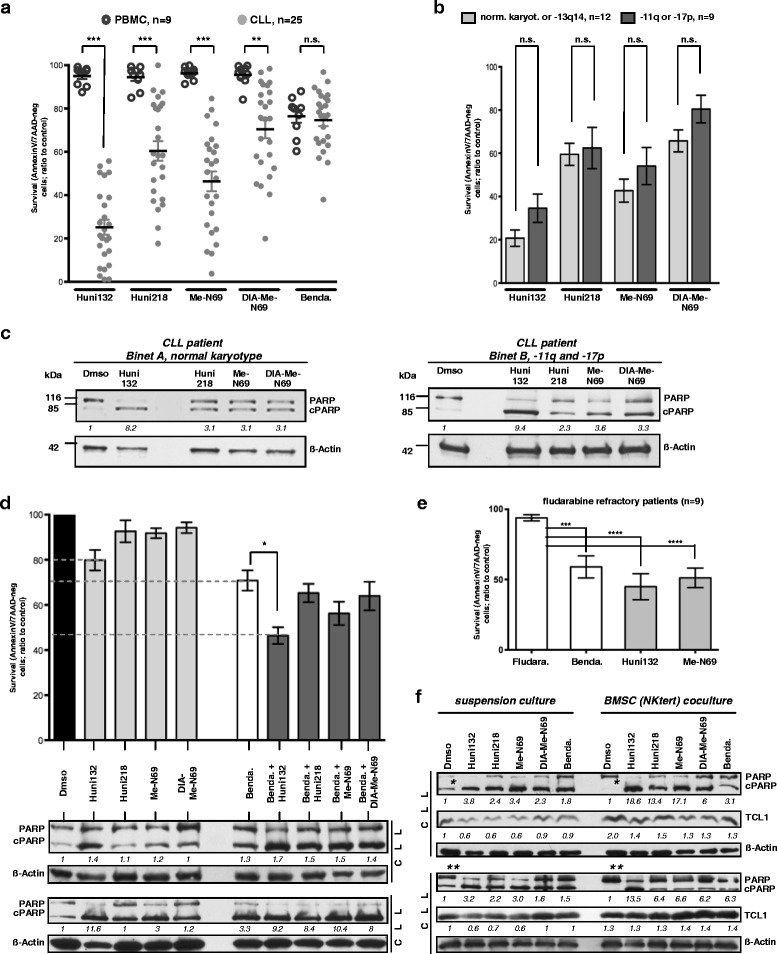
The novel organometallic nucleoside analogues (MCNA) induce marked in-vitro cell death across all cytogenetic and clinical risk subsets of CLL and can overcome stromal cell protection. Incubation (24 h) of primary CLL suspension cultures (n = 25) or healthy-donor derived PBMC (n = 9) with MCNA (10 μM) or bendamustine. Cytotoxicity as per AnnexinV/7AAD flow cytometry ((apoptotic) cell death); means/SEM. (a) Significantly (*** P<0.0001; ** P = 0.0012) higher CLL cell death induction by the MCNA (highest efficacy for Huni132) than in PBMC; a less pronounced selectivity for bendamustine. Solvent associated cell death corresponded to spontaneous death rates. (b) CLL with low-risk karyotypes (normal or isolated del13q14; n = 12) vs 9 CLL carrying the high-risk aberrations del11q or del17p (including 4 clinically fludarabine refractory patients) show no significant differences in MCNA sensitivity. (c) Immunoblot-based detection of PARP cleavage induced by MCNA in 2 CLL representing the clinical/cytogenetic profile of investigated samples (β-actin corrected densitometric values). (d) Increased in vitro cell death upon combination of MCNA (5 μM) with 25 μM bendamustine (data from 5 CLL) including a synergism for Huni132 and bendamustine (dashed lines; * P = 0.006). Bottom: corresponding levels of cleaved PARP (immunoblot, β-actin corrected densitometric values) of 2 exemplary CLL. (e) CLL B-cells from clinically fludarabine refractory patients (n = 9, including the 4 from (b); cases #32-40 in Table S2) were cultured (48 h) in the presence of fludarabine (5 μM), bendamustine (25 μM), or the 2 most efficient MCNA (10 μM). Cell viability after bendamustine (*** P = 0.0003) and especially MCNA (**** P<0.0001) was significantly reduced while lost in vitro fludarabine sensitivity paralleled the clinical resistance. (f) MCNA, especially Huni132 (10 μM), more than bendamustine (25 μM), overcome the strong pro-survival support mediated to CLL cells by cocultures with NKtert BMSC. Immunoblots depict PARP cleavage in 2 representative cases (N = 5 total). Densitometric values (β-actin corrected) for cPARP/PARP are relative to those for Dmso (set as "1") for each of the two culture conditions. BMSC coculture reduces spontaneous (Dmso) PARP cleavage (densitometry: case 1 *5.4 (suspension culture) vs 0.4 (coculture), case 2 **0.67 vs 0.07). AnnexinV/7AAD-based cell survival (not shown) paralleling these data (suspension vs coculture) was: 70.3 % vs 83.3 % (case 1) and 71.4 % vs 84 % (case 2). Increasing TCL1 levels (β-actin corrected densitometric values; only Dmso in suspension culture set as “1”) also indicate an efficient protective feeder cell interaction [26]
