Abstract
Despite benefits of using light-sensitive geolocators to track animal movements and describe patterns of migratory connectivity, concerns have been raised about negative effects of these devices, particularly in small species of aerial insectivore. Geolocators may act as handicaps that increase energetic expenditure, which could explain reported effects of geolocators on survival. We tested this ‘Energetic Expenditure Hypothesis’ in 12 populations of tree swallows (Tachycineta bicolor) and barn swallows (Hirundo rustica) from North America and Europe, using measurements of corticosterone from feathers (CORTf) grown after deployment of geolocators as a measure of physiology relevant to energetics. Contrary to predictions, neither among- (both species) nor within-individual (tree swallows only) levels of CORTf differed with respect to instrumentation. Thus, to the extent that CORTf reflects energetic expenditure, geolocators apparently were not a strong handicap for birds that returned post-deployment. While this physiological evidence suggests that information about migration obtained from returning geolocator-equipped swallows is unbiased with regard to levels of stress, we cannot discount the possibility that corticosterone played a role in reported effects of geolocators on survival in birds, and suggest that future studies relate corticosterone to antecedent factors, such as reproductive history, and to downstream fitness costs.
Keywords: Energetic Expenditure Hypothesis, feather corticosterone, hormone biomarkers, light-level geolocators, migration physiology, swallows
2. Introduction
Understanding the ecological and population processes affecting migratory birds requires knowledge of habitat use and individual movements throughout the annual cycle [1–3]. Recent insights have been facilitated by advances in techniques for tracking animal movements and describing patterns of migratory connectivity [4–7]. The use of light-sensitive geolocators has become especially popular because the devices now weigh less than 1 g and, therefore, can be used on many species of small-bodied migratory passerines [8]. Indeed, the recent rapid increase in research using geolocators has revealed previously unknown information about breeding areas [9], migratory routes and stopover areas [10,11], non-breeding areas [12–14] and migratory connectivity [9,12,15] for a variety of small bird species [8].
Despite obvious benefits of using geolocators to track migration, concerns have been raised about negative effects of these devices and the potential biases in data derived from them [16–19]. A recent meta-analysis provided evidence that geolocators can reduce survival, particularly for aerial foragers and migratory species [17]. Effects of geolocators on flight mechanics can help explain these findings and include increased wing loading and drag owing to altered aerodynamic profiles [20,21]. To compensate for these effects, individuals carrying geolocators would be expected to increase energetic expenditure [16,22,23]. This added workload could be particularly taxing during migration, which is a period of high energetic demand [22,24] and high mortality [25]. Thus, geolocators have the potential to detrimentally influence the energetic balance of migrants.
Although this ‘Energetic Expenditure Hypothesis’ may provide a reasonable mechanism for reported effects of geolocators, testing it requires measuring the energetics of free-living birds following deployment. Unlike other tracking technologies [26], current geolocators suitable for use with small birds (i.e. devices<1.0 g) cannot collect any biotelemetry data other than location. Moreover, most small migrant passerines cannot be recaptured until they return to the breeding grounds. These issues make it difficult to assess differences in en route physiology of individuals with and without geolocators, which is critical for establishing or refuting a physiological link between geolocators and variation in performance measures affecting fitness.
The hormone corticosterone (CORT) may be a useful proxy for measuring the effect of geolocators on the energetics of migratory birds. CORT is a metabolic hormone well known for its role in energy management [27,28], and CORT levels rise in response to increased energetic demands and facilitate the conversion (and thus depletion) of energy stores into usable forms [29–33]. In migratory passerines, CORT levels are elevated seasonally to meet the physiological demands of migration, but birds still respond to stressors during this period [33–35] and during winter [36,37]. Thus, if instrumentation with a geolocator acts as a handicap that unpredictably increases energetic demands, CORT levels could rise to a point where costs, such as increased catabolism of energy stores, degradation of muscle and immunosuppression occur [28,38]. Short-term effects of geolocators and other tracking devices on CORT physiology are either ephemeral (e.g. [39,40]) or not detectable (e.g. [41]), but studies of seabirds show that baseline and handling-induced CORT levels are significantly elevated the year following geolocator deployment [23,42]. All of these previous studies measured CORT during the breeding season (or in captivity) so we lack any assessment of the effects of geolocators on energetics outside of this period in wild populations.
Feathers may provide a retrospective ‘remote sensing’ of avian energetics because they contain a record of CORT during the period of feather growth [43]. The CORT in feathers (CORTf) has been shown experimentally to reflect levels of plasma CORT [44,45] and a variety of stressors [46–50] during feather growth. For birds that moult after the deployment of geolocators, CORTf could quantify a physiologically relevant proxy of energetic costs arising from instrumentation. In migratory passerines, assessing energetics during the pre-basic moult, which itself is energetically expensive [51,52], may be particularly pertinent because this moult is preceded by physiologically demanding activities (e.g. breeding, migration or both), the energetic costs of which could carry over into the moulting period. Therefore, CORTf may reflect cumulative energetic costs that could be more pronounced in birds carrying geolocators.
Using CORTf as a measure of physiological response to geolocators, we tested two predictions of the Energetic Expenditure Hypothesis: (i) compared with returning adults without geolocators from the same population (controls), individuals returning with geolocators (geolocator birds) should have higher levels of CORTf, reflecting their increased energetic expenditure; and (ii) within individuals carrying a geolocator, post-deployment levels of CORTf should be higher than pre-deployment levels. As a group, small aerial insectivores should be particularly susceptible to the effects of geolocators [17], making them appropriate models for this type of study. Thus, we tested our predictions in four populations of tree swallows (Tachycineta bicolor) and eight populations of barn swallows (Hirundo rustica) from North America and Europe. By studying how physiology of migratory passerines varies in response to geolocators, this study also provides data useful for resolving potential ethical and scientific issues facing researchers tracking small birds over long distances.
3. Material and methods
3.1. Fieldwork
Complete details of field methods, including geolocator instrumentation, for the birds in our study have been presented elsewhere (tree swallows [11,18]; barn swallows [19,53]). For tree swallows, fieldwork was conducted during May–July of 2011–2013 at three breeding sites in Canada (Prince George, British Columbia: 53°50′ N, 122°57′ W; St Denis National Wildlife Area, Saskatchewan: 52°13′ N, 106°04′ W; Long Point, Ontario: 42°39′ N, 80°26′ W) and one in the USA (Saukville, Wisconsin: 43°24′ N, 88°0′ W). Adults were captured at their nest-boxes during the brood-rearing period and individuals were banded, sexed, measured and dorsal contour feathers were collected from the upper back using forceps and stored in paper envelopes until subsequent CORT analyses. Geolocators (0.67 g; Lotek Wireless model MK12-S in 2011, MK5-S in 2012) were attached using a modified leg-loop backpack harness [10], composed of 1 mm diameter solid ethylenepropylene-diene rubber tubing, that had a combined mass of less than or equal to 1.0 g (less than 5% of body mass). The geolocator, which sat just anterior to the tail, was secured to the contour feathers on the bird's back using a small amount of cyanoacrylate adhesive and did not directly impede movement of the wings. Different adult tree swallows were marked with geolocators in 2011 and 2012.
For barn swallows, fieldwork in North America was conducted during May–July of 2012 and 2013 at two breeding sites in Canada (Prince Albert National Park, Saskatchewan: 53°42′ N, 106°3′ W; near Sackville, New Brunswick: 45°58′ N, 64°13′ W) and three in the USA (Auburn, Alabama: 32°33′ N, 85°21′ W; Greenville, Mississippi: 33°17′ N, 91°2′ W; Seattle, Washington: 47°39′ N, 122°21′ W). Fieldwork in Europe took place during April–July of 2010–2012 at one breeding site in southern Switzerland (Magadino: 46°09′ N, 8°55′ W) and two in northern Italy (Piedmont: 45°33′ N, 8°44′ E; Lombardy: 45°19′ N, 9°40′ E). Adults were captured with mist-nets, individually marked with coloured leg bands, sexed, measured and the fourth outermost tail feather (R4) was plucked and stored for CORT analysis. For North American breeding sites, geolocators (0.7 g; Migrate Technology model Intigeo-P55B1–7) were deployed at this time and were attached using a leg-loop harness composed of elastic cord (Stretch Magic, Pepperell, MA, USA). The combined mass was less than 0.8 g (approx. 4.5% of body mass). For European breeding sites, adults were recaptured at the end of the breeding season and geolocators (Swiss Ornithological Institute model SOI-GDL2.10 in 2010, SOI-GDL2.11 in 2011) were deployed. Geolocators (2010: approx. 0.77 g; 2011: approx. 0.68 g) were attached using a leg-loop harness composed of an elastic silicone rubber tubing, and the combined mass was less than 0.8 g (less than 4% of body mass).
3.2. Nomenclature and sample sizes of feathers
Feathers from geolocator birds were either grown the autumn before (pre-deployment) or after (post-deployment) deployment. The moulting of tree swallow back feathers occurs from mid-July to early November, corresponding to the beginning of autumn migration for the majority of individuals, and is probably completed within North America ([11,54,55] and references therein). Barn swallow tail feathers are moulted at the end of autumn migration on wintering grounds in Africa and South America ([55–57] and references therein).
As not all geolocator birds returned the year following deployment, for among-individual analyses of tree swallows we had four categories of feathers that comprised treatment groups: (i) feathers from controls reflecting the general population of returning individuals, (ii) post-deployment feathers from geolocator birds that returned the subsequent year, (iii) pre-deployment feathers from returning geolocator birds, and (iv) pre-deployment feathers from geolocator birds that did not return. Groups (iii) and (iv) are analogous to controls; analysing them separately enabled us to determine if differences in CORT physiology existed in these treatments prior to deployment (see Statistical analyses section). We had feathers from 40 tree swallows recaptured the year subsequent to their original sampling (control: n=12 birds; geolocator: n=28 birds). We did not have any pre-deployment feathers for barn swallows, and thus only had feathers in two treatment groups: (i) feathers from controls reflecting the general population of returning individuals and (ii) post-deployment feathers from geolocator birds that returned the subsequent year. Sample sizes for each treatment described above are presented in table 1.
Table 1.
Sample sizes of feathers from each species, population, year and sex (male/female) in each treatment group. (See text for explanation of treatments.)
| population | year | control | pre-deployment from non-returning geolocator birds | pre-deployment from returning geolocator birds | post-deployment from geolocator birds | population total |
|---|---|---|---|---|---|---|
| tree swallows | ||||||
| Long Point, ON | 2011 | 0 | 0 | 2/6 | 0 | 8 |
| 2012 | 0/1 | 0 | 0 | 6/7 | 14 | |
| 2013 | 3/5 | 0 | 0 | 2/2 | 12 | |
| total | 3/6 | 0 | 2/6 | 8/9 | 34 | |
| St Denis, SK | 2011 | 0 | 0 | 0 | 0 | 0 |
| 2012 | 4/0 | 0 | 0 | 8/2 | 14 | |
| 2013 | 0 | 0 | 0 | 0 | 0 | |
| total | 4/0 | 0 | 0 | 8/2 | 14 | |
| Prince George, BC | 2011 | 0/2 | 0 | 0/2 | 0 | 4 |
| 2012 | 5/7 | 5/11 | 5/4 | 0/1 | 38 | |
| 2013 | 5/5 | 0 | 0 | 5/5 | 20 | |
| total | 10/14 | 5/11 | 5/6 | 5/6 | 62 | |
| Saukville, WI | 2011 | 0/0 | 13/16 | 9/1 | 0 | 39 |
| 2012 | 2/8 | 0 | 0 | 9/1 | 20 | |
| total | 2/8 | 13/16 | 9/1 | 9/1 | 59 | |
| barn swallows | ||||||
| Auburn, AL | 2013 | 1/8/3a | 0 | 0 | 2/0 | 14 |
| Greenville, MS | 2013 | 7/9 | 0 | 0 | 0/1 | 17 |
| Seattle, WA | 2013 | 6/6 | 0 | 0 | 1/1 | 14 |
| Sackville, NB | 2013 | 6/9 | 0 | 0 | 0/2 | 17 |
| Prince Albert NP, SK | 2013 | 7/6 | 0 | 0 | 1/1 | 15 |
| Lombardy, IT | 2012 | 6/3 | 0 | 0 | 12/4 | 25 |
| 2013 | 2/0 | 0 | 0 | 2/0 | 4 | |
| total | 8/3 | 0 | 0 | 14/4 | 29 | |
| Piedmont, IT | 2012 | 11/3 | 0 | 0 | 11/3 | 28 |
| 2013 | 8/0 | 0 | 0 | 8/0 | 16 | |
| total | 19/3 | 0 | 0 | 19/3 | 44 | |
| Magadino, CH | 2012 | 13/3 | 0 | 0 | 13/3 | 32 |
aunknown sex.
For tree swallows, geolocator birds were randomly selected from previously banded adults. Control birds were selected as the next banded adult captured, which was generally the same day or shortly after deployment of a geolocator, so control and geolocator birds were well matched in their timing of breeding. For barn swallows, in 2010, birds were assigned alternately to control or geolocator groups within each colony of each breeding site. In 2011, this procedure was maintained at the Magadino and Piedmont breeding sites, but at the Lombardy site different breeding colonies were assigned to different treatment groups for practical reasons. Regardless, in both years and at all sites, birds in the two treatment groups were well matched in their timing of breeding.
3.3. Analysis of corticosterone from feathers
Analyses of CORTf were conducted as in previous studies of tree swallows [44,58]. We first processed feathers by removing the calamus, weighing and measuring the length of the remaining portion of feather, placing each sample into a separate glass vial, and cutting the samples into small pieces with scissors. We then added 10 ml of HPLC-grade methanol (VWR International, Mississauga, Ontario, Canada) to each sample, sonicated all samples at room temperature for 30 min, followed by incubation at 50°C overnight in a water bath. A vacuum filtration system consisting of a plug of polyester wool in a glass filtration funnel was used to separate the methanol extract from the feather material. The original sample vial, remnant feather pieces and filtration material were washed twice with approximately 2.5 ml of additional methanol that was then added to the original methanol extract. Methanol extracts were placed in a 50°C water bath and subsequently evaporated in a fume hood. Samples were extracted in six batches. Recovery efficiency of the methanol extraction was assessed by including feather samples spiked with approximately 5000 CPM of 3H-labelled CORT, and an average of 93.4% (s.d.=6.1) of the radioactivity was recoverable in the reconstituted samples. Samples were adjusted for recoveries. Extract residues were reconstituted in a small volume of phosphate buffer (0.05 mol l−1, pH 7.6) and analysed by radioimmunoassay in duplicate following [59]. Serial dilutions of sample extracts of both species were parallel to the standard curve, indicating no interference with the antibody. All samples were run blind with regard to individual identity. Samples from all populations except Saukville, WI, were randomly distributed throughout five assays, and the average intra-assay variability, computed using three aliquots per assay of the same standard, was 8.8% (s.d.=5.4), inter-assay variability was 9.1%, and all samples were above the limit of detection (ED80; average±s.d.: 16.08±2.42 pg 100 μl−1). Saukville samples were obtained 1 year later and randomized throughout a single assay run with a different internal standard but same antiserum as all previous samples. Our statistical analyses do not compare CORTf values among populations (we intentionally control for this using population as a random effect; see Statistical analyses section) and are instead tested for differences among treatments within sites. This single assay had an intra-assay variability of 5.7% (i.e. was internally valid) and all samples were above its limit of detection (ED80) of 12.99 pg 100 μl−1 (similar to the other assays). CORTf values were standardized by feather length (i.e. CORT mm−1) to best represent the time-dependent deposition of CORT [43,60,61].
3.4. Statistical analyses
CORTf values were log-transformed to improve normality. We used mixed models to analyse the effect of geolocators on CORTf, using Proc Mixed in SAS v. 9.2 (SAS Institute, Cary, NC, USA), including population and year as random effects to account for clustered data and annual effects. Owing to the unbalanced sample sizes among treatment, sex, year and population, all mixed models used the Kenward-Rogers method for approximating degrees of freedom. Non-significant interaction terms (p>0.05) were eliminated from models. Because different types of feathers were used for tree swallows and barn swallows, we analysed each species separately.
For both species, we first examined the variation in CORTf among treatments. These models started with fixed effects of treatment (for definitions see Nomenclature and sample sizes section), minimum age (youngest reliably estimable age) and sex, and included a treatment×sex interaction. Only known-sex birds were used in analyses that included sex. Second, for tree swallows alone, we addressed within-individual effects of instrumentation with a geolocator using the 40 individuals sampled in two consecutive years. Each bird was used as its own control by subtracting pre-deployment (year 1) values from post-deployment (year 2) values. This created a single variable that reflected the within-individual change in CORT physiology from one year to the next. We compared this variable between geolocator and control birds in a model that also included the fixed effects of minimum age and sex, and a treatment×sex interaction.
4. Results
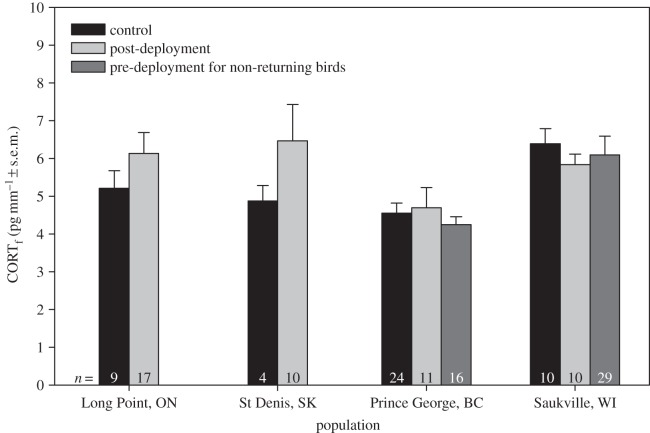
In tree swallows, there was no interaction between treatment and sex on CORTf (F3,152=0.22, p=0.88), so the interaction term was removed from the model. The final model revealed no effect of geolocators on CORTf (F3,149=0.69, p=0.56; figure 1), and no effect of sex (F1,154=1.14, p=0.29) or minimum age (F8,153=0.45, p=0.89). In barn swallows, there was no interaction between treatment and sex on CORTf (F1,169=0.07, p=0.79), so this term was also removed from the model. The final model showed no effect of geolocators on CORTf (F1,171=0.47, p=0.49; figure 2), and no effect of sex (F1,170=2.45, p=0.12) or age (F5,170=0.47, p=0.80).
Figure 1.
Levels of corticosterone in feathers (CORTf) from tree swallows instrumented with a geolocator compared to non-instrumented (control) birds. All feathers were grown post-breeding, and pre-deployment feathers were grown the year prior to deployment of geolocators. See text for complete descriptions of treatments. Note that some populations contain multiple years of data (table 1).
Figure 2.
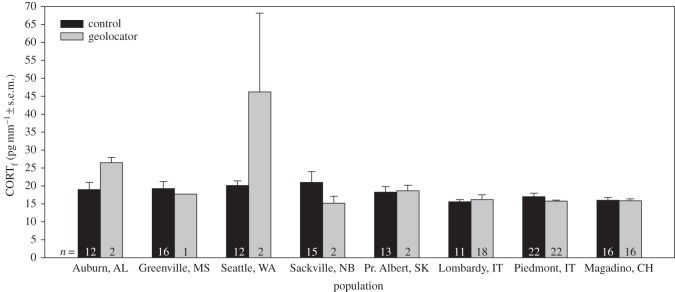
Levels of corticosterone in feathers (CORTf) from barn swallows instrumented with a geolocator compared to controls. All feathers were grown post-breeding (i.e. after deployment of geolocators). Note that some populations contain multiple years of data (table 1).
When we considered the 40 cases where tree swallows were sampled in two consecutive years, we found that within-individual changes in CORTf were not related to the interaction of treatment with sex (F1,30=1.80, p=0.19) so this term was removed from the model. The final model revealed no effect of geolocators on within-individual changes in CORTf from one year to the next (F1,31=0.28, p=0.60; figure 3), and no effects of age (F6,31=0.27, p=0.95) or sex (F1,31=0.53, p=0.47).
Figure 3.
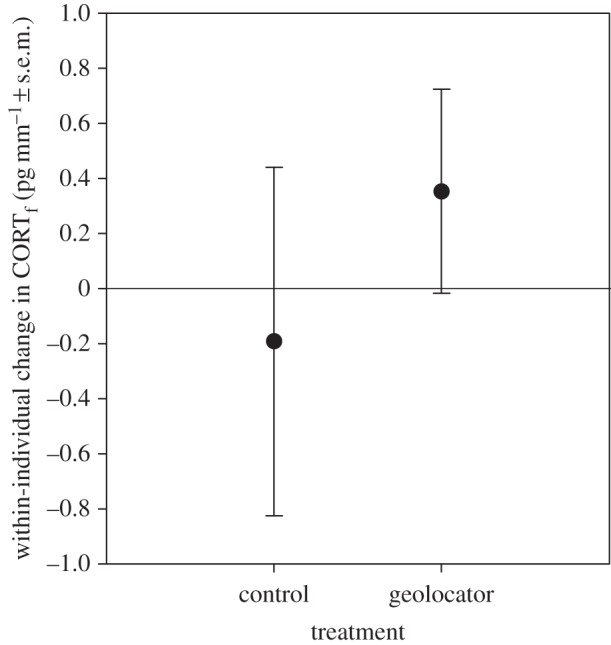
Within-individual change in levels of corticosterone from feathers (CORTf) of tree swallows in two consecutive years. For control birds (n=12), this is the change in levels from year 1 to year 2; for geolocator birds (n=28), this reflects the change from pre-deployment (year 1) to post-deployment (year 2) levels.
5. Discussion
We tested the Energetic Expenditure Hypothesis that geolocators attached to aerial insectivores produce a handicap that increases energetic demand. We predicted that if there was a pervasive effect of geolocators it would be reflected in levels of CORT from feathers grown prior to or early-on during post-breeding migration (tree swallows), or at the end of migration (barn swallows). We also expected that our broad geographical and temporal approach of analysing 3 years of CORTf data from 12 populations of two species of aerial insectivore on two continents would provide the power to detect an effect of geolocators if one existed. However, our results based on both among-individual (both species) and within-individual (tree swallows only) analyses show that there was no effect of geolocators on levels of CORTf. The lack of effect in barn swallows is particularly revealing, considering that they carried the geolocator for considerably longer before moulting than did tree swallows. Thus, to the extent that CORTf reflects energetic expenditure, our findings suggest that geolocators apparently did not act as a strong handicap for birds that returned post-deployment. We further speculate that this provides physiological evidence that data about locations and timing of migration obtained from returning geolocator birds (e.g. [11]) may not be biased with regard to levels of stress, although this should be tested directly.
Our findings do not rule out, however, an effect of geolocators on CORT physiology, nor do they necessarily discount CORT as a potential mediator of the effects of geolocators on survival. If non-returning geolocator birds had CORT physiology operating in homeostatic overload (sensu [62]) for extended periods, then they could have experienced costs including reduced condition, increased susceptibility to disease or death (for reviews see [28,38]). Sub-lethal effects of CORT could have reduced the ability of these birds to acquire resources during stopovers or on the wintering grounds, or influenced their decision to not travel as far as controls, resulting in lower return rates to breeding grounds the subsequent year, which have been detected in several of our populations [18,19]. Moreover, factors operating prior to departure from the breeding grounds could have predisposed non-returning geolocator birds to potential negative effects arising from instrumentation. For example, reproductive effort can influence CORT physiology during and at the end of the breeding season [63,64] which, in turn, can have consequences for migration phenology [65,66]. If reproduction was particularly energetically demanding for non-returning geolocator birds (i.e. CORT levels were already near homeostatic overload), then geolocators could have further increased CORT levels and exacerbated costs. Although behavioural data indicate that control and geolocator tree swallows do not appear to differ immediately after instrumentation [18], physiological costs could have carried over into migration which would further increase energetic demands. The duration, speed and distance of the migratory journey, as well as habitat use during stopovers and on wintering grounds, can influence energetics, CORT physiology and return rates of birds [36,37,67–69]. Indeed, migration distance is believed to influence apparent survival rates of geolocator-marked birds [17], and CORTf could possibly predict the pace of autumn and spring migration in tree swallows and barn swallows, respectively [65]. Thus, the ecophysiological context before, during and after migration is important for fully understanding how and when geolocators influence survival, the potential fitness consequences to survivors, and the extent to which CORT physiology is involved in these processes.
Regardless of the mechanism, individuals that were better able to manage their CORT physiology may have been better able to avoid costs [62] and thus survive. Measuring CORT from feathers grown post-deployment from non-returning geolocator birds is essential to substantiating this hypothesis but is not possible owing to difficulties recapturing swallows once they leave the breeding grounds. Thus, our ability to identify any obvious physiological differences between returning and non-returning geolocator birds is limited to comparing their pre-deployment CORTf levels with controls, yet we found no differences among these three groups. Investigation of plasma CORT at the time of deployment should be a focus of future research. Although we lack evidence of physiological differences between returning and non-returning geolocator birds, it may be the case that only high-quality birds were instrumented to begin with, and this explains why CORTf levels of returning geolocator birds did not differ from controls. This is a possibility for tree swallows because geolocators were deployed (albeit randomly) on previously banded birds that had already survived at least two migrations, but we can rule out this hypothesis for barn swallows because deployment of geolocators was completely randomized [19]. Nonetheless, it is important to note that, despite surviving and not having significantly higher levels of CORTf, returning geolocator birds may still have incurred a cost of instrumentation. Indeed, initial evidence in European populations of barn swallows suggests that geolocators impair subsequent reproduction [19]. It is unknown what role CORT plays in such effects, so future studies would benefit from determining whether body condition, health, or reproductive variables the spring following instrumentation vary with respect to CORTf in returning geolocator birds.
Additional research is clearly needed to identify if physiological costs of instrumentation with geolocators exist and whether these influence survival, and the Energetic Expenditure Hypothesis provides testable predictions of such effects. To the extent that we can use CORTf to infer variation in energetic expenditure, our results suggest that geolocators may not have imposed a handicap on returning swallows. Moreover, compared with birds that did not return and breed, returning birds did not have significantly different CORTf in the year prior to instrumentation. Whether or not only the best-quality birds survived to be sampled and how CORT physiology may have contributed to this require future research. Longitudinal demographic studies such as ours are particularly informative for addressing how CORTf relates to antecedent factors, such as reproductive history, and to downstream fitness costs. Validation studies are needed to determine if the levels of energetic exertion (or physiological stress) necessary to influence CORTf are similar among species. As our understanding of migratory movements and stopover areas improve (e.g. [11]), it will become easier to sample geolocator and control birds throughout migration. Comparing physiological profiles of these birds at multiple stages throughout their journey will be essential to substantiating or refuting the Energetic Expenditure Hypothesis.
Acknowledgements
We thank S. Gray for the use of her laboratory, kindly appreciate the logistical support of H. de la Giroday and D. Frattinger, and thank S. Cabezas for her help in the laboratory.
Ethics statement
All feathers were collected in accordance with appropriate ethics permits in North America (University of Saskatchewan 20070041 and 20100084; University of Northern British Columbia ACUC-2011-13; University of Guelph 11R042; Tulane University 0387) and Europe (Office fédéral de l'environnement, Division Espèces, écosystèmes, paysages F044-0799; Regione Lombardia no. 329 and no. 2141; Provincia di Novara no. 905).
Data accessibility
Data for this study have been deposited with Dryad ().
Funding statement
This study was funded by Bird Studies Canada (D.W.B.), the Canadian Foundation for Innovation (D.R.N.), Environment Canada (R.G.C., K.A.H.), EU INTERREG program (project ID 157624065), Fondazione Cariplo (grant no. UNIAGI 13357 to N.S.), Milan University (grant no. 2009-ATE-0015 to D.R.), the Natural Sciences and Engineering Research Council of Canada (D.R.N., D.W.B., R.D.D., R.G.C., L.L.B.), the University of Guelph (D.R.N.), the University of Milano-Bicocca (grant no. 2011-ATE-0272 to R.A.), the University of Northern British Columbia (R.D.D., L.L.B.), Tulane University (C.M.T.) and the National Science Foundation (DEB-0933602 to C.M.T.). The Swiss federal office for environment contributed financial support for the development of the data loggers (UTF-Nr. 254, 332, 363, 400).
Author contributions
R.G.C. initially conceived the study, with subsequent contributions from R.D.D., D.R.N., K.A.H., N.S. and C.M.T.; all authors contributed to data collection in the field; G.D.F. and L.L.B. conducted the corticosterone analyses in T.A.M.'s laboratory; G.D.F. analysed the data and wrote the manuscript with all authors providing input; all authors have approved the final version of the manuscript.
Conflict of interests
We have no competing interests.
References
- 1.Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. (doi:10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 2.Webster MS, Marra PP. 2005. The importance of understanding migratory connectivity and seasonal interactions. In Birds of two worlds: the ecology and evolution ofmigration (eds Greenberg R, Marra PP), pp. 199–209. Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- 3.Norris DR, Marra PP. 2007. Seasonal interactions, habitat quality, and population dynamics in migratory birds. Condor 109, 535–547. (doi:10.1650/8350.1) [Google Scholar]
- 4.Hobson KA, Wassenaar LI (eds). 2008. Tracking animal migration with stable isotopes. New York, NY: Academic Press. [Google Scholar]
- 5.Webster MS, Marra PP, Haig SM, Bensch S, Holmes RT. 2002. Links between worlds: unraveling migratory connectivity. Trends Ecol. Evol. 17, 76–83. (doi:10.1016/s0169-5347(01)02380-1) [Google Scholar]
- 6.Robinson WD, Bowlin MS, Bisson I, Shamoun-Baranes J, Thorup K, Diehl RH, Kunz TH, Mabey S, Winkler DW. 2010. Integrating concepts and technologies to advance the study of bird migration. Front. Ecol. Environ. 8, 354–361. (doi:10.1890/080179) [Google Scholar]
- 7.Ambrosini R, Møller AP, Saino N. 2009. A quantitative measure of migratory connectivity. J. Theor. Biol. 257, 203–211. (doi:10.1016/j.jtbi.2008.11.019) [DOI] [PubMed] [Google Scholar]
- 8.McKinnon EA, Fraser KC, Stutchbury BJ. 2013. New discoveries in landbird migration using geolocators, and a flight plan for the future. Auk 130, 211–222. (doi:10.1525/auk.2013.12226) [Google Scholar]
- 9.Seavy NE, Humple DL, Cormier RL, Gardali T. 2012. Establishing the breeding provenance of a temperate-wintering North American passerine, the golden-crowned sparrow, using light-level geolocation. PLoS ONE 7, 34886 (doi:10.1371/journal.pone.0034886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stutchbury BJM, Tarof SA, Done T, Gow E, Kramer PM, Tautin J, Fox JW, Afanasyev V. 2009. Tracking long-distance songbird migration by using geolocators. Science 323, 896 (doi:10.1126/science.1166664) [DOI] [PubMed] [Google Scholar]
- 11.Laughlin AJ. 2013. Integrating information from geolocators, weather radar, and citizen science to uncover a key stopover area of an aerial insectivore. Auk 130, 230–239. (doi:10.1525/auk.2013.12229) [Google Scholar]
- 12.Bairlein F, Norris DR, Nagel R, Bulte M, Voigt CC, Fox JW, Hussell DJ, Schmaljohann H. 2012. Cross-hemisphere migration of a 25 g songbird. Biol. Lett. 8, 505–507. (doi:10.1098/rsbl.2011.1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser KC. 2012. Continent-wide tracking to determine migratory connectivity and tropical habitat associations of a declining aerial insectivore. Proc. R. Soc. B 279, 4901–4906. (doi:10.1098/rspb.2012.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quillfeldt P, Masello JF, Navarro J, Phillips RA. 2013. Year-round distribution suggests spatial segregation of two small petrel species in the South Atlantic. J. Biogeogr. 40, 430–441. (doi:10.1111/jbi.12008) [Google Scholar]
- 15.Ryder TB, Fox JW, Marra PP. 2011. Estimating migratory connectivity of gray catbirds (Dumetella carolinensis) using geolocator and mark-recapture data. Auk 128, 448–453. (doi:10.1525/auk.2011.11091) [Google Scholar]
- 16.Barron DG, Brawn JD, Weatherhead PJ. 2010. Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 1, 180–187. (doi:10.1111/j.2041-210X.2010.00013.x) [Google Scholar]
- 17.Costantini D, Møller AP. 2013. A meta-analysis of the effects of geolocator application on birds. Curr. Zool. 59, 697–706 [Google Scholar]
- 18.Gómez J, Michelson CI, Bradley DW, Norris DR, Berzins LL, Dawson RD, Clark RG. 2014. Effects of geolocators on reproductive performance and annual return rates of a migratory songbird. J. Ornithol. 155, 37–44. (doi:10.1007/s10336-013-0984-x) [Google Scholar]
- 19.Scandolara C. 2014. Impact of miniaturized geolocators on barn swallow Hirundo rustica fitness traits. J. Avian Biol. 45, 417–423. (doi:10.1111/jav.00412) [Google Scholar]
- 20.Pennycuick C, Fast PL, Ballerstädt N, Rattenborg N. 2012. The effect of an external transmitter on the drag coefficient of a bird's body, and hence on migration range, and energy reserves after migration. J. Ornithol. 153, 633–644. (doi:10.1007/s10336-011-0781-3) [Google Scholar]
- 21.Bowlin MS, Henningsson P, Muijres FT, Vleugels RH, Liechti F, Hedenström A. 2010. The effects of geolocator drag and weight on the flight ranges of small migrants. Methods Ecol. Evol. 1, 398–402. (doi:10.1111/j.2041-210X.2010.00043.x) [Google Scholar]
- 22.Bowlin MS, Cochran WW, Wikelski MC. 2005. Biotelemetry of New World thrushes during migration: physiology, energetics and orientation in the wild. Integr. Comp. Biol. 45, 295–304. (doi:10.1093/icb/45.2.295) [DOI] [PubMed] [Google Scholar]
- 23.Quillfeldt P, McGill RA, Furness RW, Möstl E, Ludynia K, Masello JF. 2012. Impact of miniature geolocation loggers on a small petrel, the thin-billed prion Pachyptila belcheri. Mar. Biol. 159, 1809–1816. (doi:10.1007/s00227-012-1971-0) [Google Scholar]
- 24.Wikelski M, Tarlow EM, Raim A, Diehl RH, Larkin RP, Visser GH. 2003. Avian metabolism: costs of migration in free-flying songbirds. Nature 423, 704 (doi:10.1038/423704a) [DOI] [PubMed] [Google Scholar]
- 25.Sillett TS, Holmes RT. 2002. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 71, 296–308. (doi:10.1046/j.1365-2656.2002.00599.x) [Google Scholar]
- 26.Cooke SJ, Hinch SG, Wikelski M, Andrews RD, Kuchel LJ, Wolcott TG, Butler PJ. 2004. Biotelemetry: a mechanistic approach to ecology. Trends Ecol. Evol. 19, 334–343. (doi:10.1016/j.tree.2004.04.003) [DOI] [PubMed] [Google Scholar]
- 27.Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. 1993. Feast and famine: critical role of glucocorticoids with insulin in daily energy-flow. Front. Neuroendocrinol. 14, 303–347. (doi:10.1006/frne.1993.1010) [DOI] [PubMed] [Google Scholar]
- 28.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89. (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 29.DuRant SE, Hopkins WA, Hepp GR, Romero LM. 2013. Energetic constraints and parental care: is corticosterone indicative of energetic costs of incubation in a precocial bird?? Horm. Behav. 63, 385–391. (doi:10.1016/j.yhbeh.2012.12.001) [DOI] [PubMed] [Google Scholar]
- 30.Harvey S, Phillips JG, Rees A, Hall TR. 1984. Stress and adrenal function. J. Exp. Zool. 232, 633–645. (doi:10.1002/jez.1402320332) [DOI] [PubMed] [Google Scholar]
- 31.Dallman MF, Akana SF, Pecoraro NC, Warne JP, la Fleur SE, Foster MT. 2007. Glucocorticoids, the etiology of obesity and the metabolic syndrome. Curr. Alzheimer Res. 4, 199–204. (doi:10.2174/156720507780362236) [DOI] [PubMed] [Google Scholar]
- 32.Landys MM, Ramenofsky M, Guglielmo CG, Wingfield JC. 2004. The low-affinity glucocorticoid receptor regulates feeding and lipid breakdown in the migratory Gambel's white-crowned sparrow Zonotrichia leucophrys gambelii. J. Exp. Biol. 207, 143–154. (doi:10.1242/jeb.00734) [DOI] [PubMed] [Google Scholar]
- 33.Landys MM, Ramenofsky M, Wingfield JC. 2006. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 148, 132–149. (doi:10.1016/j.ygcen.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 34.Landys-Ciannelli MM, Ramenofsky M, Piersma T, Jukema J, Wingfield JC. 2002. Baseline and stress-induced plasma corticosterone during long-distance migration in the bar-tailed godwit, Limosa lapponica. Physiol. Biochem. Zool. 75, 101–110. (doi:10.1086/338285) [DOI] [PubMed] [Google Scholar]
- 35.Holberton RL, Parrish JD, Wingfield JC. 1996. Modulation of the adrenocortical stress response in neotropical migrants during autumn migration. Auk 113, 558–564. (doi:10.2307/4088976) [Google Scholar]
- 36.Marra PP, Holberton RL. 1998. Corticosterone levels as indicators of habitat quality: effects of habitat segregation in a migratory bird during the non-breeding season. Oecologia 116, 284–292. (doi:10.1007/s004420050590) [DOI] [PubMed] [Google Scholar]
- 37.Holberton RL, Able KP. 2000. Differential migration and an endocrine response to stress in wintering dark-eyed juncos (Junco hyemalis). Proc. R. Soc. Lond. B 267, 1889–1896. (doi:10.1098/rspb.2000.1226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone-behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206. (doi:10.1093/icb/38.1.191) [Google Scholar]
- 39.Suedkamp Wells KM, Washburn BE, Millspaugh JJ, Ryan MR, Hubbard MW. 2003. Effects of radio-transmitters on fecal glucocorticoid levels in captive dickcissels. Condor 105, 805–810. (doi:10.1650/7174) [Google Scholar]
- 40.Pereira RJG, Granzinolli MAM, De Barros FM, Duarte JMB. 2009. Influence of radiotransmitters on fecal glucocorticoid levels of free-ranging male American kestrels. J. Wildl. Manage. 73, 772–778. (doi:10.2193/2008-184) [Google Scholar]
- 41.Tremblay Y, Cherel Y, Oremus M, Tveraa T, Chastel O. 2003. Unconventional ventral attachment of time–depth recorders as a new method for investigating time budget and diving behaviour of seabirds. J. Exp. Biol. 206, 1929–1940. (doi:10.1242/jeb.00363) [DOI] [PubMed] [Google Scholar]
- 42.Elliott KH, McFarlane-Tranquilla L, Burke CM, Hedd A, Montevecchi WA, Anderson WG. 2012. Year-long deployments of small geolocators increase corticosterone levels in murres. Mar. Ecol. Prog. Ser. 466, 1–7. (doi:10.3354/meps09975) [Google Scholar]
- 43.Bortolotti GR, Marchant TA, Blas J, German T. 2008. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 22, 494–500. (doi:10.1111/j.1365-2435.2008.01387.x) [Google Scholar]
- 44.Fairhurst GD, Marchant TA, Soos C, Machin KL, Clark RG. 2013. Experimental relationships between plasma- and feather-levels of corticosterone in a free-living bird. J. Exp. Biol. 216, 4071–4081. (doi:10.1242/jeb.091280) [DOI] [PubMed] [Google Scholar]
- 45.Hõrak P, Männiste M, Meitern R, Sild E, Saks L, Sepp T. 2013. Dexamethasone inhibits corticosterone deposition in feathers of greenfinches. Gen. Comp. Endocrinol. 191, 210–214. (doi:10.1016/j.ygcen.2013.07.002) [DOI] [PubMed] [Google Scholar]
- 46.Fairhurst GD, Frey MD, Reichert JF, Szelest I, Kelly DM, Bortolotti GR. 2011. Does environmental enrichment reduce stress? An integrated measure of corticosterone from feathers provides a novel perspective. PLoS ONE 6, 17663 (doi:10.1371/journal.pone.0017663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fairhurst GD, Navarro J, González-Solís J, Marchant TA, Bortolotti GR. 2012. Feather corticosterone of a nestling seabird reveals consequences of sex-specific parental investment. Proc. R. Soc. B 279, 177–184. (doi:10.1098/rspb.2011.0884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meitern R, Sild E, Lind MA, Manniste M, Sepp T, Karu U, Horak P. 2013. Effects of endotoxin and psychological stress on redox physiology, immunity and feather corticosterone in greenfinches. PLoS ONE 8, 67545 (doi:10.1371/journal.pone.0067545) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harriman VB, Dawson RD, Clark RG, Fairhurst GD, Bortolotti GR. 2014. Effects of ectoparasites on seasonal variation in quality of nestling tree swallows (Tachycineta bicolor). Can. J. Zool. 92, 87–96. (doi:10.1139/cjz-2013-0209) [Google Scholar]
- 50.Will AP, Suzuki Y, Elliott KH, Hatch SA, Watanuki Y, Kitaysky AS. 2014. Feather corticosterone reveals developmental stress in seabirds. J. Exp. Biol. 217, 2371–2376. (doi:10.1242/jeb.098533) [DOI] [PubMed] [Google Scholar]
- 51.Klaassen M. 1995. Moult and basal metabolic costs in males of two subspecies of stonechats: the European Saxicola torquata rubicula and the East African S. t. axillaris. Oecologia 104, 424–432. (doi:10.1007/BF00341339) [DOI] [PubMed] [Google Scholar]
- 52.Lindström Å, Visser GH, Daan S. 1993. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 66, 490–510 [Google Scholar]
- 53.Liechti F. In press Timing of migration and residence areas during the non-breeding period of barn swallows Hirundo rustica in relation to sex and population. J. Avian Biol. (doi:1111/jav.00485) [Google Scholar]
- 54.Stutchbury BJ, Rohwer S. 1990. Molt patterns in the tree swallow (Tachycineta bicolor). Can. J. Zool. 68, 1468–1472. (doi:10.1139/z90-217) [Google Scholar]
- 55.Rohwer S, Butler LK, Froehlich DR. 2005. Ecology and demography of east-west differences in molt scheduling of neotropical migrant passerines. In Birds of two worlds: the ecology and evolution of migration (eds Greenberg R, Marra PP), pp. 87–105. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 56.Saino N, Romano M, Caprioli M, Ambrosini R, Rubolini D, Scandolara C, Romano A. 2012. A ptilochronological study of carry-over effects of conditions during wintering on breeding performance in the barn swallow Hirundo rustica. J. Avian Biol. 43, 513–524. (doi:10.1111/j.1600-048X.2012.05622.x) [Google Scholar]
- 57.García-Peréz B, Hobson KA. 2014. A multi-isotope (δ2H, δ13C, δ15N) approach to establishing migratory connectivity of barn swallow (Hirundo rustica). Ecosphere 5, 21 (doi:10.1890/es13-00116.1) [Google Scholar]
- 58.Fairhurst GD, Treen GD, Clark RG, Bortolotti GR. 2012. Nestling corticosterone response to microclimate in an altricial bird. Can. J. Zool. 90, 1422–1430. (doi:10.1139/cjz-2012-0096) [Google Scholar]
- 59.Blas J, Baos R, Bortolotti GR, Marchant T, Hiraldo F. 2005. A multi-tier approach to identifying environmental stress in altricial nestling birds. Funct. Ecol. 19, 315–322. (doi:10.1111/j.1365-2435.2005.00976.x) [Google Scholar]
- 60.Bortolotti GR, Marchant T, Blas J, Cabezas S. 2009. Tracking stress: localisation, deposition and \hboxstability of corticosterone in feathers. J. Exp. Biol. 212, 1477–1482. (doi:10.1242/jeb.022152) [DOI] [PubMed] [Google Scholar]
- 61.Bortolotti GR. 2010. Flaws and pitfalls in the chemical analysis of feathers: bad news-good news for avian chemoecology and toxicology. Ecol. Appl. 20, 1766–1774. (doi:10.1890/09-1473.1) [DOI] [PubMed] [Google Scholar]
- 62.Romero LM, Dickens MJ, Cyr NE. 2009. The reactive scope model: a new model integrating homeostasis, allostasis, and stress. Horm. Behav. 55, 375–389. (doi:10.1016/j.yhbeh.2008.12.009) [DOI] [PubMed] [Google Scholar]
- 63.Bonier F, Moore IT, Robertson RJ. 2011. The stress of parenthood? Increased glucocorticoids in birds with experimentally enlarged broods. Biol. Lett. 7, 944–966. (doi:10.1098/rsbl.2011.0391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Done T, Gow EA, Stutchbury BJM. 2011. Corticosterone stress response and plasma metabolite levels during breeding and molt in a free-living migratory songbird, the wood thrush (Hylocichla mustelina). Gen. Comp. Endocrinol. 171, 176–182. (doi:10.1016/j.ygcen.2011.01.006) [DOI] [PubMed] [Google Scholar]
- 65.Stutchbury BJM, Gow EA, Done T, MacPherson M, Fox JW, Afanasyev V. 2011. Effects of post-breeding moult and energetic condition on timing of songbird migration into the tropics. Proc. R. Soc. B 278, 131–137. (doi:10.1098/rspb.2010.1220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultner J, Moe B, Chastel O, Tartu S, Bech C, Kitaysky AS. 2014. Corticosterone mediates carry-over effects between breeding and migration in the kittiwake Rissa tridactyla. Mar. Ecol. Prog. Ser. 496, 125–133. (doi:10.3354/meps10603) [Google Scholar]
- 67.Klaassen M. 1994. Growth and energetics of tern chicks from temperate and polar environments. Auk 111, 525–544 [Google Scholar]
- 68.Marra PP, Holmes RT. 2001. Consequences of dominance-mediated habitat segregation in American redstarts during the nonbreeding season. Auk 118, 92–104. (doi:10.1642/0004-8038(2001)118[0092:codmhs]2.0.co;2) [Google Scholar]
- 69.Angelier F, Holberton RL, Marra PP. 2009. Does stress response predict return rate in a migratory bird species? A study of American redstarts and their non-breeding habitat. Proc. R. Soc. B 276, 3545–3551. (doi:10.1098/rspb.2009.0868) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study have been deposited with Dryad ().