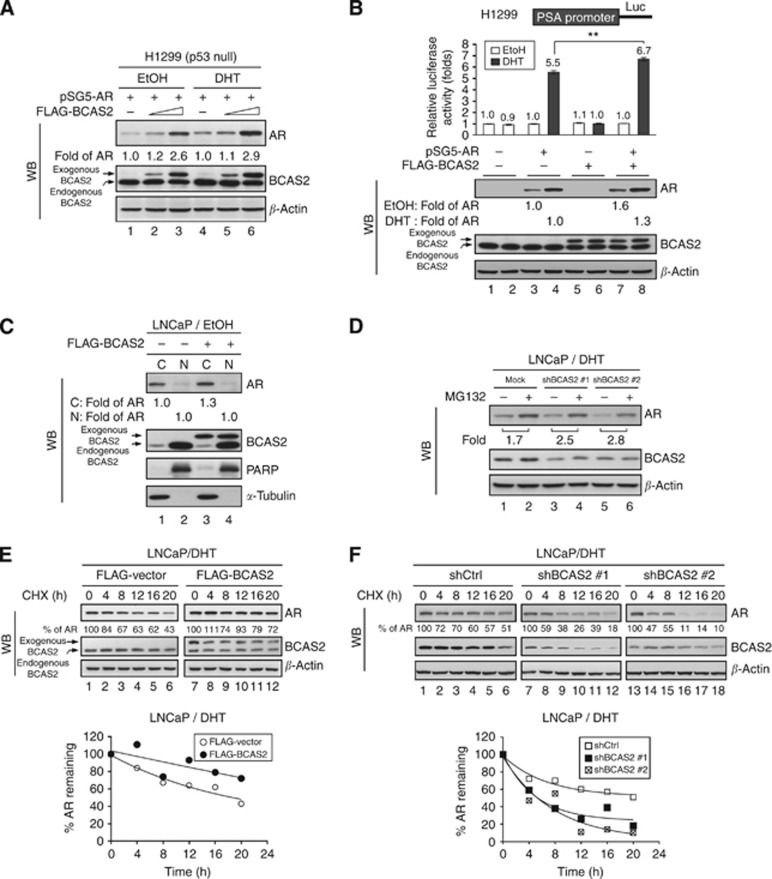
Figure 4.
BCAS2 increases AR protein expression via a p53-independent proteasome pathway and enhances AR protein stability. (A) BCAS2 enhances AR protein expression in p53 null cells. H1299 cells were transiently transfected with AR along with increasing doses of FLAG-BCAS2 plasmid. (B) Overexpression of BCAS2 increases AR-dependent transcriptional activity. Upper, luciferase assays. Lower, WB. **: Student's t-test, P<0.01. (C) BCAS2 has no effect on AR nuclear translocation. Without ligand treatment, LNCaP cells overexpressing BCAS2 were harvested for N/C fractionation. Blots were probed for AR, BCAS2, PARP (for the nuclear fraction control), and α-tubulin (cytosolic fraction). Data from nuclear fractions were normalised to PARP; cytosolic fractions were normalised to α-tubulin. (D) BCAS2 protects AR from proteasome degradation. LNCaP cells transfected with shBCAS2#1 or shBCAS2#2 were treated with MG132 (20 μM) or equal volume DMSO (vehicle for MG132) in the presence of DHT. (E) Overexpression of BCAS2 increases AR protein stability. Cells transfected with FLAG-BCAS2 were incubated with CHX (200 μg ml−1) for the times indicated. The percentage of AR indicates the amount of AR at each time point, relative to the control (time 0 set as 100%). Upper panel, WB. Lower panel, the calculated regression curves. (F) Depletion of BCAS2 reduces AR protein stability. LNCaP cells transfected with shCtrl or two different shBCAS2 constructs in the presence of DHT were subjected to CHX-chase assay as described in panel E.