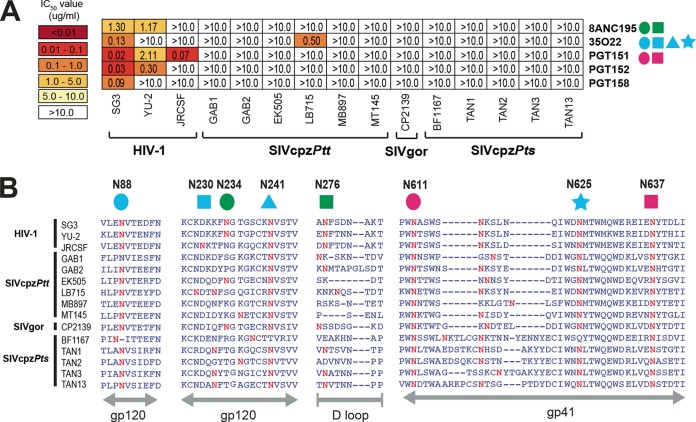
FIG 4 .
Neutralizing capacity of antibodies targeting the interface of HIV-1 gp120 and gp41 regions. (A) The ability of glycan-associated antibodies (right) to neutralize HIV-1, SIVcpz, and SIVgor strains (bottom) is shown. Numbers indicate IC50s (in micrograms per milliliter) from TZM-bl cells, averaged from three different experiments, with a heat map indicating the relative neutralization potency. Colored shapes to the right of each antibody indicate the N-linked glycans that are associated with antibody neutralizing activity. Antibody 8ANC195 contacts N234 (green circle) and N276 (green square), 35O22 utilizes N88 (blue circle), N230 (blue square), N241 (blue triangle), and N625 (blue square), and PGT151 requires N611 (pink circle) and N637 (pink square) for optimal neutralization (53–56). The highest antibody concentration used was 10 µg/ml. (B) Conservation of glycans associated with antibody neutralizing activity. An alignment of HIV-1, SIVcpz, and SIVgor Env protein sequences is shown, with predicted N-linked glycans (NXS/T) highlighted in red. N-linked glycans known to influence bNab binding are highlighted above the alignment, with HXB2 numbering in black. The positions of various Env regions are shown in gray below the alignment.