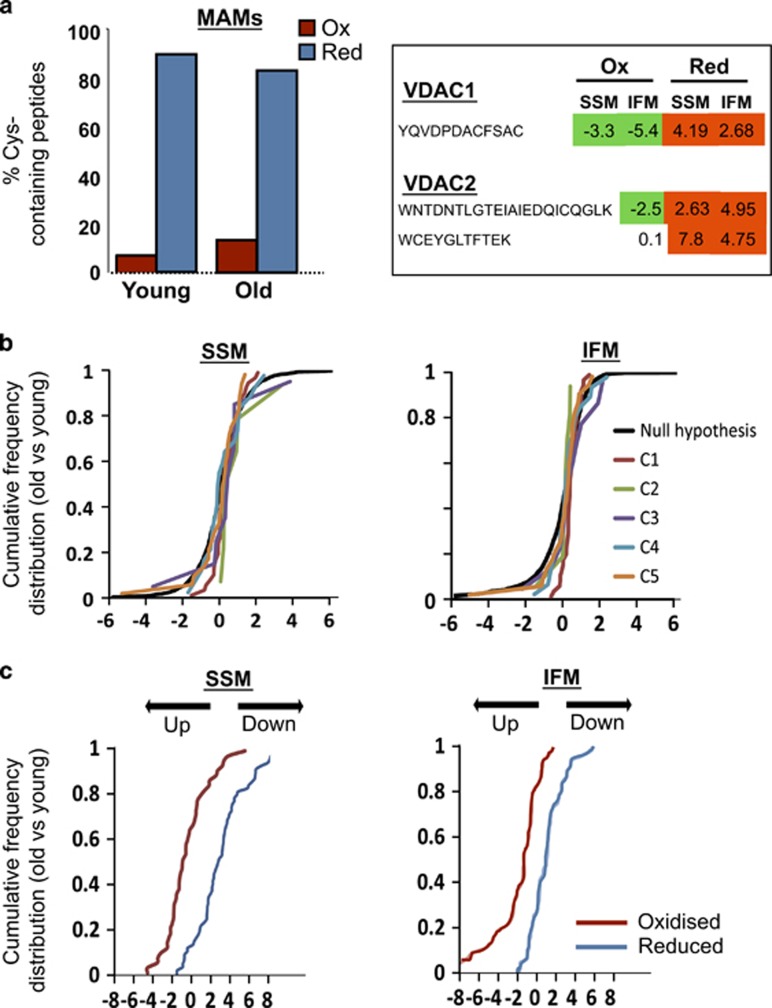
Figure 6.
Effect of aging on: (a) quantitative redox proteomics using GELSILOX of proteins from mitochondria-associated membranes (MAMs; left) and of the Cys-containing peptides detected in mitochondrial VDAC proteins (right). The left graph represents the percentage of Cys-containing peptides identified in oxidized (red) or reduced (blue) forms. The numbers in the right table represent the standardized variable at the peptide level as in b; negative values indicate an increase (in green) and positive values a decrease (in red) in the peptides containing Cys in oxidized form (Ox) or in reduced form (Red) in mitochondrial VDAC proteins in hearts from old mice. (b) The abundance of mitochondrial respiratory complexes (1–5) in subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria. Data are shown as cumulative distributions of the standardized variable at the protein level (i.e., corrected log2 ratios of proteins expressed in units of standard deviation) for all oxidative phosphorylation proteins. The black sigmoid is the theoretical null hypothesis distribution; a displacement toward the left indicates an increase in protein concentration. All the categories follow very closely the null hypothesis distribution, indicating that aging does not affect the abundance of mitochondrial respiratory proteins. (c) Alterations in the abundance of oxidized (red) and reduced (blue) Cys-containing peptides in SSM and IFM from young and old mice hearts. Peptides containing Cys residues in different oxidation states were quantified using the GELSILOX method. The sigmoid curves represent the cumulative distribution of the standardized variable at the peptide level (i.e. corrected log2-ratios of peptides expressed in units of S.D.), for all peptides containing either oxidized or reduced Cys sites that belong to proteins from OxPhos complexes