FIGURE 7:
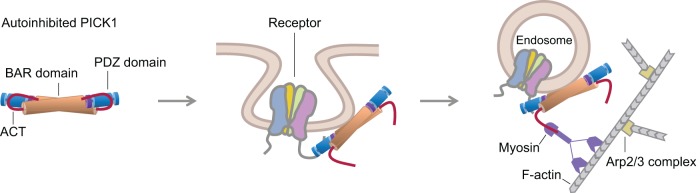
Model of PICK1 function in receptor endocytosis and trafficking. Published evidence and data presented here support a model for PICK1 involvement in receptor endocytosis. PICK1 exists in an autoinhibited state in which its membrane-binding surface is masked by interactions of the ACT with the PDZ and BAR domains. Binding of the PDZ domain to receptor tails at the membrane triggers a conformational change that exposes the membrane-binding capacity of PICK1 (Madsen et al., 2008). We propose that this conformational change consists of a movement of the ACT away from the contiguous membrane-binding surface formed by the BAR and PDZ domains. Analogous to other BAR domain proteins (Saheki and De Camilli, 2012), PICK1 may participate in the endocytic pathway by generating or sensing membrane curvature while also linking to the cytoskeleton for vesicle movement. Consistently our results suggest that the conformational change in PICK1 frees the ACT for interaction (direct or indirect) with a motility factor to propel vesicle movement. We propose that this motility factor is myosin, which is consistent with the fact that myosins are the major drivers of synaptic transport (Kneussel and Wagner, 2013).
