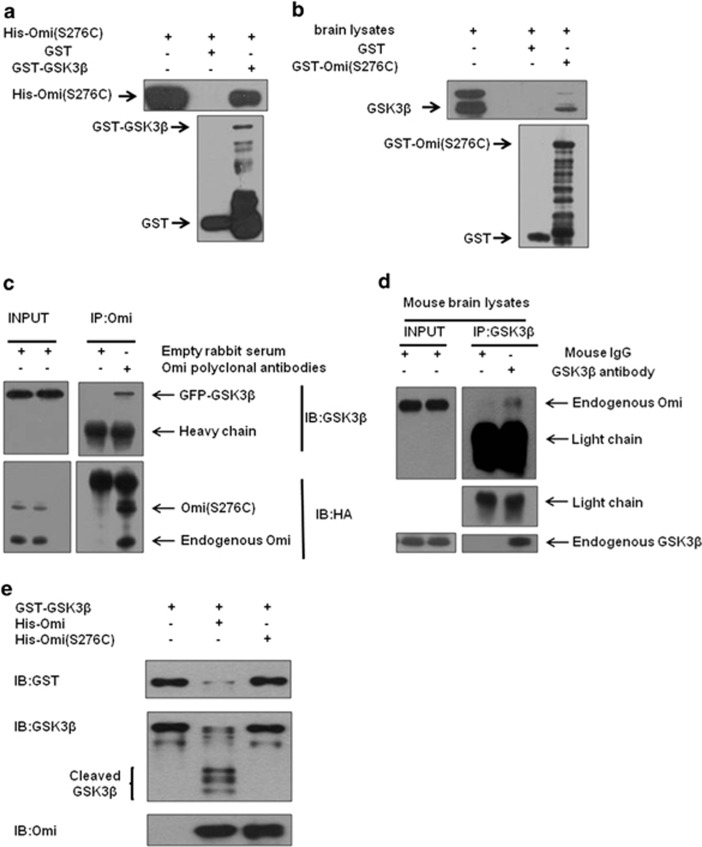
Figure 4.
GSK3β is a substrate of Omi. (a) In vitro pulldown assays were performed showing that GST-GSK3β but not GST alone, interacted with His-Omi. Three independent experiments were performed. (b) The semi in vivo pulldown assays were performed showing that GST-S276C mutant Omi but not GST alone, pulled down endogenous GSK3β from brain lysates. Three independent experiments were performed. (c) Immunoprecipitation assays were performed showing that Omi interacted with GSK3β in N2a cells. Three independent experiments were performed. (d) Immunoprecipitation assays showed that endogenous Omi interacted with endogenous GSK3β in the brain of mice. Three independent experiments were performed. (e) In vitro cleavage assays showed that GST-GSK3β was cleaved by His-Omi, but not by His-Omi S276C. In vitro purified GST-GSK3β was incubated with WT or protease-inactive S276C Omi for 60 min in protease buffer at 37 °C. The incubated mixtures were subjected to western blotting analysis with an anti-GST or anti-GSK3β antibody. The brace indicates proteolytic fragments. Three independent experiments were performed