Abstract
The intrarenal renin-angiotensin-system (RAS) plays a critical role in the pathogenesis and progression of hypertension. In angiotensin (Ang) II-dependent hypertension, collecting duct renin synthesis and secretion are stimulated despite suppression of juxtaglomerular (JG) renin. This effect is mediated by Ang II type 1 receptor (AT1R) independently of blood pressure. While the regulation of JG renin has been extensively studied, the mechanisms by which renin is regulated in the collecting duct remain unclear. The augmentation of renin synthesis and activity in the collecting duct may provide a pathway for additional generation of intrarenal and intratubular AngII formation due to the presence of angiotensinogen substrate and angiotensin converting enzyme in the nephron. The recently described (pro)renin receptor ((P)RR) binds renin or prorenin enhancing renin activity and fully activating the biologically inactive prorenin peptide. We have recently described that renin and (P)RR, are augmented in the renal inner medulla and urine in rats infused with Ang II. However, the functional contribution of (P)RR to enhance renin activity in the collecting duct, and the contribution to increased intrarenal Ang II content and development of hypertension, have not been elucidated. In this review we will focus on the recent evidence demonstrating the mechanism by which renin is regulated in the collecting ducts and its interaction with the (P)RR. Recent evidence suggests that renin and (P)RR interact to contribute to Ang II formation and the stimulation of intracellular pathways in the renal collecting duct which may contribute to the development and progression of hypertension.
Keywords: Angiotensin II dependent hypertension, renin angiotensin system, collecting duct, renin, prorenin receptor
Introduction
The renin-angiotensin system (RAS) plays a major role in the blood pressure and fluid volume control; however, it also potentially contributes to the development and progression of fibrotic and hypertrophic diseases. Beside the systemic RAS, which is mainly controlled by the production and release of renin from the kidneys into the circulation, locally acting RAS present in a variety of organs are also important particularly in the kidney. Renin is primarily synthesized in the juxtaglomerular (JG) cells where is stimulated by a number of signals that increase intracellular cyclic AMP (cAMP) levels and activation of protein kinase A (PKA). The 5′-flanking non-coding region of the renin gene (1;2) plays a central role in regulating renin expression in all species. This region is considered as a classical promoter. The presence of a functional cAMP response element (CRE) is a characteristic feature of the renin promoter in all species; cAMP binds to the two regulatory subunits of PKA to release two catalytic subunits from the inactive PKA tetramer complex. The free catalytic subunits (referred to as activated PKA) translocate to the nucleus and phosphorylate transcription factors of the cAMP response element binding protein/activating transcription factor CREB. In contrast to the stimulatory function of cAMP, hormones such as Ang II and endothelin inhibit renin gene expression and secretion from JG cells by increasing the cytosolic calcium and/or activation of PKC (3).
New evidence from at least 10 years, demonstrated the presence of prorenin and renin in renal collecting ducts (4-11). Furthermore, it has been reported that Ang II increases renin expression in the collecting duct cells in vitro and in vivo via PKC pathway (12). This is opposite to what has been observed in JG cells (3). This observation was relevant in the view that angiotensinogen (AGT) and angiotensin converting enzyme (ACE) are also present along the nephron and upregulated by Ang II-infusions (13;14), indicating that augmented collecting duct renin may contribute to further intratubular Ang II formation.
Recently, a new member of the RAS has been described: the (pro)renin receptor ((P)RR), which is able to bind renin enhancing renin activity and fully activating the biologically inactive prorenin (15;16), which may lead to a further Ang II formation inside the kidneys (17). Additionally, binding of prorenin and renin to the membrane bound (P)RR triggers intracellular pathways which have been related to tissue damage (18;19). In this review, we will describe evidence demonstrating that collecting duct principal cells synthesize renin and prorenin and that this cell type respond to Ang II increasing renin/prorenin synthesis and release. We suggest that renin/prorenin - (P)RR interaction may have a key role in the formation of intrarenal Ang II.
Intratubular RAS along the nephron
The concentrations of Ang I and Ang II in the proximal tubule fluid are in the range of 5-10 pmol/ml (20-22), which are similar to renal interstitial fluid concentrations (23) and remain elevated in infused hypertensive rats (24) suggesting their sustained actions on proximal reabsorption rate. The presence of AT1R on luminal membranes of proximal and distal nephron segments suggested that Ang II concentrations are able to activate AT1R (25;26). In mice, AT1aR are essential for normal blood pressure regulation and for mediating the hypertensive response to Ang II infusions (27). These recent studies indicate that distal nephron Ang II is formed locally in the tubules at concentrations that are sufficiently high to influence distal nephron transport function (25;26). Angiotensinogen (AGT) mRNA has been described in proximal tubules (13;28;29). This observation generated interest about its function. A breakthrough was the publication of several reports describing the augmentation of mRNA and urinary excretion in chronic Ang II infusion model (28;29). This effect is mediated via activation of AT1R, since AT1R blockers were able to prevent AGT upregulation (30). Similar observations were made in vitro using proximal tubule cell cultures (31). The mechanism by which Ang II stimulates AGT mRNA and protein is complex and appears to require interactions with inflammatory factors including interleukin (32), and increased oxidative stress (33;34). Augmented AGT may serve as substrate for Ang I formation by the action of renin in the collecting duct.
Renin in the collecting duct
Although renin is primarily synthesized and secreted by the JG cells, renin mRNA and protein expression is also present in renal tubules (5-7;10;11). Figure 1A shows renin expression in JG cells and collecting ducts. Renin synthesis is augmented in the principal cells of connecting tubules and cortical and medullary collecting ducts of chronic Ang II-infused rats (Figure 1, B, C, D) and mice, Cyp1a1-Ren2 transgenic rats, and in both kidneys of two-kidney one clip Goldblatt hypertensive rats. (5-7;10;11). In contrast to the inhibitory effect that Ang II exerts on JG renin, Ang II stimulates renin in the principal collecting duct cells via a mechanism mediated by the AT1R independent of changes in blood pressure (5-7). Activation of AT1R suppresses renin synthesis in JG cells via protein kinase C (PKC) and Ca+2 (35); however, we demonstrated that augmentation of renin synthesis in rat inner medullary collecting duct cells is mediated directly by AT1R via a PKC pathway (12). The PKC activator, PMA (Phorbol 12-myristate 13-acetate) stimulated renin levels and concomitant treatment of 10−7M Ang II and PKC inhibition with calphostin C prevented the stimulatory effect of Ang II on collecting duct renin in inner medullary collecting duct (IMCD) cells (35).
Figure 1.
A. Renin expression in juxtaglomerular cells (insert in red and arrow) and in collecting ducts (red). Brown color indicates aquaporin-2 (AQP-2) immunostaining, which is a specific marker for principal cells of the collecting duct. B. Renin immunolabeling in collecting ducts of a control rat; C. Renin is augmented in collecting ducts of Ang II infused rats. D. In Angiotensin II infused rats AT1 receptor antagonist blunted this effect. (modified from Prieto-Carrasquero et al., 2004; 2005).
Because AGT and angiotensin converting enzyme (ACE) are present along the nephron, the augmented of renin levels in the collecting duct from Ang II-dependent hypertensive rats may explain why newly Ang II is formed intratubularly in this animal model of hypertension. Furthermore, there is recent evidence suggesting that high intrarenal Ang II levels can be explained not only by uptake of existing Ang II but also by newly formed Ang II (36;37). These mechanisms are explained by the presence of all the components required to form Ang II inside the kidneys, like angiotensinogen, renin and angiotensin converting enzyme (9). More importantly, these observations suggest that intra-renal and intratubular Ang II can act directly to enhance sodium reabsorption thus contributing to the development and maintenance of hypertension (25;26).
The (Pro)renin receptor
The discovery of a receptor for renin and prorenin, the prorenin receptor ((P)RR) brings a new perspective of the possible role of (P)RR in a setting of activated intra-renal RAS. The (P)RR is expressed in the kidneys particularly in mesangial cells, podocytes, and intercalated type-A cells of distal nephron segments (38-41). The (P)RR was described as an associated protein with the v-ATPase (vacuolar H+-ATPase), giving the name to the gene ATP6AP2 (ATPase 6 accessory protein 2/(P)RR). The (P)RR binds renin and prorenin with an affinity in the nanomolar range and their binding triggers a range of cellular events like mitogen activated kinases (MAPKs) extracellular-signal-regulated kinases (ERK 1/2). Importantly, the actions of the (P)RR has been linked to diabetes nephropathy (42). Despite the low levels of renin in patients with diabetic nephropathy, high levels of prorenin is detected in these patients associated with occurrence of microvascular complications, microalbuminuria and retinopathy (43). The (P)RR can activate inflammatory responses in diabetic kidneys, for example overexpression of (P)RR leads to augmentation of COX-2 (44). Blockade of prorenin binding to the (P)RR is able to prevent and even reverse diabetic nephropathy (45). Synthesis of prorenin is also augmented in the collecting ducts in diabetic nephropathy, leading to activation of signalling pathways that can promote tubular damage (46). All this new evidence suggest the interaction between the (P)RR and renin or prorenin in plasma, interstitial space or in tubular fluids where they may contribute to the generation of additional Ang II levels. In fact, prorenin levels in the plasma are not correlated with plasma renin activity in some diseases (43), which suggest complex interactions depending on the status of prorenin or (P)RR expression. We have demonstrated that the (P)RR is augmented in the collecting ducts of rats infused with Ang II during 14 days (41), however the pattern between mRNA and post-transcriptional events seems to be different, making the (P)RR-renin/prorenin interaction complex especially in diabetic nephropathy and hypertension.
Interestingly, binding of renin and prorenin to the (P)RR can induces a four-fold increase in the catalytic efficiency of angiotensinogen conversion to Ang I (18;47). Thus, renin/prorenin interaction with its receptor may provide a novel mechanism for tissue Ang I generation on the cell surface. As shown in Figure 2A, (P)RR (apical) is co-expressed with the anion exchanger type 1 (AE-1; basolateral membrane) in intercalated cells while renin is expressed in principal cells (Figure 2B)
Figure 2.
Prorenin receptor, Anion Exchanger-1 and renin in normal rat kidney tissues. A. (Pro)renin receptor (arrows, green; apical cell side) is co-expressed with anion exchanger type 1 (arrow head, red; basolateral cell side), a marker for intercalated cells. B. (Pro)renin receptor is present in intercalated cells (green) while renin is expressed in principal cells (red, as noticed by the positive control of positive renin juxtaglomerular cells, arrow). Augmented renin/prorenin and (pro)renin receptor in Ang II-dependent hypertensive rats suggest that an interaction between both components may contribute to intratubular Ang I and Ang II formation and activation of PRR signaling pathways. Blue: DAPI; 4′,6-diamidino-2-phenylindole, nuclei marker.
Expression of Renin and (P)RR in collecting duct cells
We have demonstrated that urines collected from Ang II infused rats incubated with recombinant prorenin showed high Ang I formation that correlates with the augmentation of the soluble form of the (P)RR (s(P)RR) and the augmentation of the (P)RR mRNA levels (41). This suggests that the augmented renin synthesis and release in the principal cells of the collecting ducts can stimulate the membrane located (P)RR leading to both, Ang I formation and/or activating (P)RR-dependent signalling pathways. Using a mouse cortical collecting duct cell line, M-1 cells, we detected the presence of renin in principal cells and (P)RR in intercalated cells (Figure 3). We also reported that (P)RR mRNA and protein expression are increased in the renal medulla of Ang II-infused rats and Cyp1a1Ren2 transgenic rat model (inducible form of Ang II-dependent malignant hypertension) (48). In addition, we also demonstrated that the soluble form of the (P)RR (s(P)RR) which is the extracellular cleaved form of the full length protein, is augmented in inner medullary tissues and in the urine of rats with Ang II-dependent hypertension (Figure 4), and more importantly that, the soluble form is functionally active in the urine (41). The s(P)RR is part of the extracellular domain of the (P)RR and corresponds to the cleaved form of the full length (37 kDa) protein and can be detected at around 28 kDa. In vitro evidence have demonstrated that activation of furin (a protease) is responsible of the cleavage of the full length form of the (P)RR, since furin specific inhibitors abolish this effect. The soluble form of the (P)RR can be detected in plasma and urine as we previously reported (41;49).
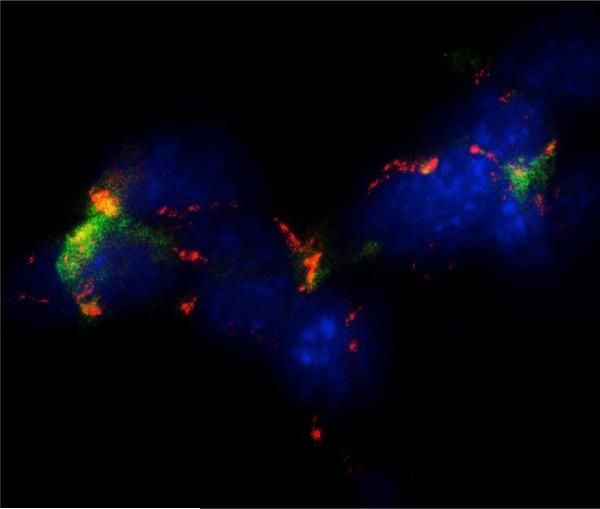
Figure 3.
Immunocytochemical evidence of the expression of renin in principal cells (red) and (pro)renin receptor (green) in intercalated cells in M-1 collecting duct cell line. Blue (DAPI; 4′,6-diamidino-2-phenylindole) is a nuclei marker. The image demonstrates close interaction between principal cells; the source of renin and prorenin and intercalated cells that expressing the (pro)renin receptor.
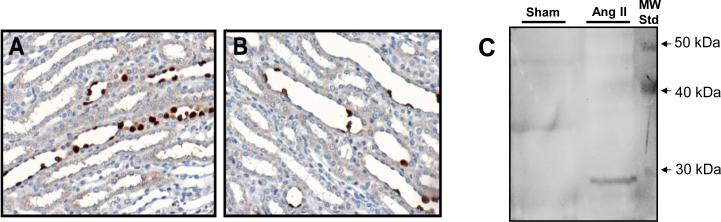
Figure 4.
Expression of the (P)RR in medullary collecting ducts in normal rats (A) and Ang II infused rats for 14 days. Ang II infusion causes a decrease in the number of positive cells. Despite decrease in cells with immunoreactivity for (pro)renin receptor bound to the membrane, the soluble cleaved form of the (pro)renin receptor was augmented in medullary tissues and importantly detected in the urine of the Ang II infused rats (C). Ang II infusion for 14 days also causes the augmentation of renin and prorenin in the collecting ducts suggesting that (pro)renin receptor and renin can interact to contribute to Ang I formation and further Ang II generation (modified from Gonzalez et al., 2011).
The increases in collecting duct renin, (P)RR gene expression and s(P)RR activity may have a key role in mediating local augmentation of intrarenal angiotensin peptides content and intratubular Ang II de novo generation, since there is plenty angiotensin converting enzyme activity in the distal nephron segments and urine (26;50;51) Importantly, increased intrarenal Ang II content contributes to the pathogenesis of chronic hypertension through sustained stimulation of Na+ reabsorption, renal vasoconstriction, and development of renal injury (9). We have suggested that (P)RR activation could be an effect of the agonist action of CD prorenin/renin which is also locally augmented by Ang II (Ref? I think you can cite here the Pflugler article).
The evidence of the secretion of the s(P)RR reported by us (41) and the in vitro data showing that the extracellular domain can be released by a furin-mediated mechanism (49), suggest that s(P)RR can bind renin secreted by the principal collecting duct cells and trigger additional formation of Ang I. Increased mRNA and protein levels of prorenin reported in freshly isolated rat primary cultures of rat inner medullary collecting duct cells in response to Ang II stimulation (12) and the evidence showing upregulation of s(P)RR, suggest that interaction between these two components (41).
(P)RR and Cyclooxygenase-2
Due to the demonstrations that (P)RR activation upregulates cyclooxygenase type 2 (COX-2) via ERK 1/2 pathways (44) contributing to inflammatory process in renal cortex, is it also important to evaluate what are the effects of (P)RR stimulation and overexpression in distal nephron segments and interstitial cells. We have recently reported that activation of the (P)RR) using a recombinant prorenin is able to increase ERK ½ phosphorylation in primary cultured inner medullary cells composed of principal collecting duct cells, intercalated cells and interstitial cells (52). We have also shown that (P)RR activation increases COX-2 expression (Ref). This is important in the view that the inner medulla COX-2 metabolites play a crucial role in the maintenance of sodium/water balance and appropriate vascular tone responses during RAS activations, thus, activation of COX-2 and synthesis of COX-2 metabolites may have a role not only in inflammatory responses but also in the maintenance of the buffer mechanism against the anti-natriuretic effects of RAS activation.
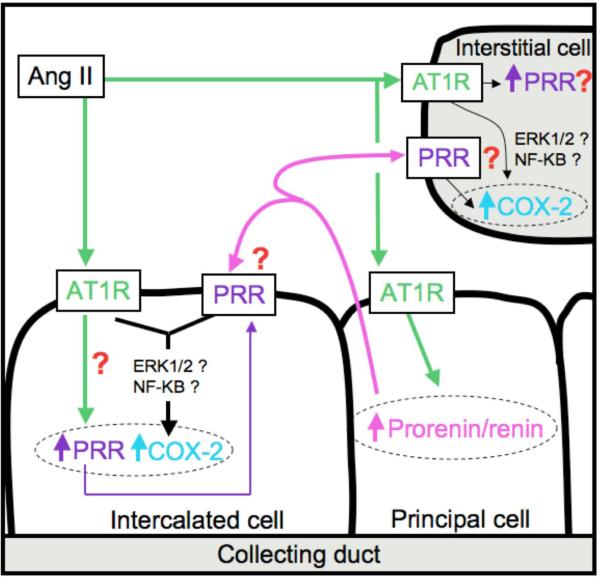
As hypothesized in Figure 5, activation of (P)RR in intercalated cells and interstitial cells by augmented secretion of prorenin and renin from the principal cells of the collecting increases intracellular pathways leading to COX-2 upregulation (Ref). Furthermore, due to the high expression of intrarenal ACE (53) and the presence of AGT (13;54)in proximal tubules, it is possible that augmented renin expression due to AT1R activation will result in a positive loop increasing intratubular Ang II de novo formation.
Figure 5.
Hypothetic role the (pro)renin receptor in collecting ducts and intercalated cells in response to Ang II. Ang II stimulates prorenin and renin synthesis in principal cells of the collecting ducts as well as (P)RR levels. The (P)RR activates ERK 1/2 pathways stimulating COX-2 expression.
Discussion
The present data and our previous reports demonstrate the close related interaction between (pro)renin, renin and the (P)RR and their role during Ang II-dependent hypertension. Angiotensin II for 14 days in male Sprague-Dawley rats resulted in increases in renal (P)RR transcript levels in the collecting duct, as well as augmentation of the soluble form in renal inner medullary tissues and urine. In addition, we have shown that (P)RR immunoreactivity was detected not only at the apical aspect of the type A intercalated cells, but also on the nonA-nonB intercalated cells. These observations in vivo are of relevance in light of recent demonstrations of renin upregulation in distal nephron segments of Ang II-dependent hypertensive rats (5-7), and renin and/or (pro)renin secretion by collecting duct cells (12); altogether providing a mechanistic basis for enhanced tubular Angiotensin II formation in hypertension.
Besides the reported augmentation of angiotensinogen in proximal tubules, renin and prorenin/renin in the collecting ducts, and Ang II levels in kidney tissues during Ang II-dependent hypertension, the (P)RRs seems to be a new member candidate for targeting the intrarenal RAS dysregulation. Nguyen and associates originally reported the presence of (P)RR predominantly in glomerular mesangial cells and in vascular smooth muscle cells of renal arteries using immunofluorescence on frozen kidney tissues (55). Our findings confirms demonstrate the predominant immunoexpression of (P)RR on the apical side of type A intercalated cells of cortical and medullary collecting ducts, based upon its colocalization in cells that express anion exchanger type 1 (Figure 2). Specific immunoexpression of (P)RR by the intercalated type A cells was reported previously (56). We showed specific (P)RR immunoreactivity on the apical aspect of intercalated cells at the initial part of the cortical collecting duct and connecting tubule, co-localizing with glycoprotein ammonia transporter glycoprotein C and B (Rhcg, and Rhbg) expressed at the basolateral cell-side of type nonA-nonB intercalated cells (Figure 6). The co-localization and homology of (P)RR with an accessory protein of the H+ -ATPase along with the demonstration that Ang II may activate vacuolar H+ -ATPase, thereby being able to add H+ into the urine at the end of the nephron; have led to suggest that the (P)RR might be involved in H+ transport via a local Ang II-dependent mechanism. Thus, the presence of (P)RR in the intercalated cells may not only contribute to the pool of angiotensin II content but also to urine acidification.
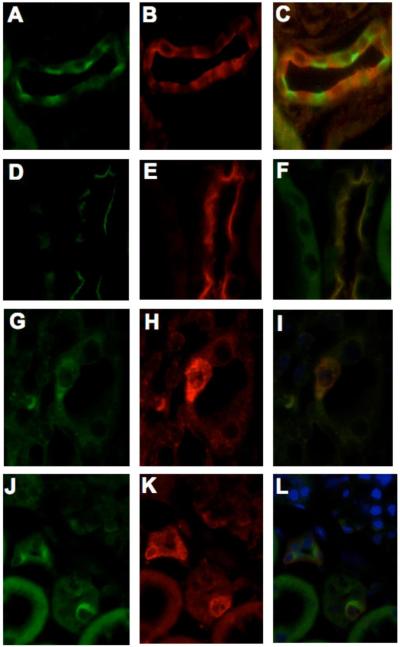
Figure 6.
Specific cell type localization of the (P)RR in the collecting ducts of rat kidneys. (P)RR positive cells showed specific immunostaining at the apical side (A) in green color, the absence of merging colors (C) indicates that (P)RR receptor is not co-localized with aquaporin-2 (B; red). The co-localization of apical (pro)renin receptor (D, G, J; green,) with apical Rhcg (E, H; red) or with basolateral Rhbg (K; red) is demonstrated in panels F, I and L, respectively. D, E, F: cortex; G, H, I, J, K, L: medulla.
Although we did not visualize (P)RR immunoexpression at the interstitial side (basolateral membrane) of the intercalated cells in vivo, Nguyen et al., described the immunoexpression of the (P)RR on the basolateral side of distal tubular cells as well as in macula densa cells (17). This particular cell-side localization of the (P)RR might be also important for regulating Ang II levels in the renal interstitium. Increases in renal interstitial fluid angiotensin II levels have been reported in several models of Ang II-dependent hypertension (57;58). Because it has been suggested that local regulation of Ang II formation in the renal interstitial compartment as well as enhanced production of interstitial Ang II might be secondary to specialized Ang II-forming pathways or accumulation mechanisms, the role of (P)RR in the regulation of renal interstitial fluid Ang II may be also important.
We have been also demonstrated by immunoblotting analysis that the protein levels of the full length (P)RR (37 kDa band) are substantially decreased in the renal medulla of chronic Ang II-infused rats (41). In contrast, these rats exhibited increased protein levels of the s(P)RR in these tissues, suggesting that post transcriptional changes in the intracellular processing of the (P)RR can be induced by a mechanism dependent on Ang II (41). Recently, it has been shown that s(P)RR can be subcellularly generated by a furin-mediated cleavage (49). Indeed, we demonstrated that furin protein levels were increased in the renal medulla of chronic Ang II-infused rats, indicating that in this model of experimental hypertension, the cleavage and apical secretion of the s(P)RR can be induced. These observations were also confirmed in in vitro experiments were Ang II was able to increase the s(P)RR in culture media, suggesting that this is an intracellular mechanism activated by AT1R (41).
The present review supports the notion that in the chronic Ang II-dependent hypertensive rats the physical interaction between (P)RR and renin expressed in neighbour principal and intercalated cells on the collecting duct contribute to increase intratubular Ang II due to its location at the apical aspect of the intercalated cells by binding renin or prorenin produced and secreted by the neighbouring principal cell, anchoring renin and/or prorenin at the cell surface helping to prevent or minimize renin washout into the urine and also increasing renin activity and the secretion of the s(P)RR form into the urine where it may bind renin, thereby enhancing even further the intraluminal conversion of angiotensinogen to Ang I, and ultimately to Ang II.
As shown by our co-immunoprecipitation studies, the s(P)RR form is bound to renin in the urine of rats chronically infused; providing in vivo functional evidence that the s(P)RR in the lumen of distal segments by binding renin may enhance the endogenous intratubular production of Ang II. Indeed, we found that the chronic Ang II-infused rats exhibited higher urinary renin activity and Ang II levels than controls. Recently, Shao et al., reported that increased Ang II levels in the kidneys and urine of rats during chronic Ang II infusion involved stimulation of endogenous intrarenal Ang II formation since intrarenal and urine Ile5-Ang II contents were greater in Val5-Ang II-infused rats than in sham-operated rats; indicating that in this model of hypertension there is stimulation of endogenous Ang II formation which contributes to overall augmentation of intrarenal Ang II (36;37).
Conclusions
The existence of an inducible form of the (P)RR in renal medullary tissues and urine and the augmented synthesis and secretion of renin in chronic Ang II-infused rats support further the importance that this interaction may have on the enhancement of intratubular renin activity during pathophysiological conditions. The recent in vivo demonstrations that increased urinary Ang II concentrations in mice infused chronically with Ang II enhance distal sodium reabsorption (59;60) emphasize further the importance that renin and (P)RR interaction may have in the distal nephron segments for the increases in intratubular Ang II formation, thus allowing for a greater distal tubular sodium reabsorption and leading to progression of hypertension. Further studies are necessary to establish the mechanisms by which Ang II enhances (P)RR synthesis and postranscriptional processing, as well as to elucidate the real contribution of the full length and the soluble form of the (P)RR to the increases of intrarenal Ang II generation.
Acknowledgments
Source of funding
Research reported in this publication was supported by Fondecyt, Chile (Award #11121217 for A.A.G), the National Institute of General Medical Sciences of the National Institutes of Health under Award #P30GM103337 (for M.C.P), and the Tulane School of Medicine Pilot Funds (for M.C.P).
Reference List
- 1.Borensztein P, Germain S, Fuchs S, Philippe J, Corvol P, Pinet F. cis-Regulatory elements and trans-acting factors directing basal and cAMP-stimulated human renin gene expression in chorionic cells. Circ Res. 1994;74:764–73. doi: 10.1161/01.res.74.5.764. [DOI] [PubMed] [Google Scholar]
- 2.Castrop H, Hocherl K, Kurtz A, Schweda F, Todorov V, Wagner C. Physiology of kidney renin. Physiol Rev. 2010 Apr;90(2):607–73. doi: 10.1152/physrev.00011.2009. [DOI] [PubMed] [Google Scholar]
- 3.Muller MW, Todorov V, Kramer BK, Kurtz A. Angiotensin II inhibits renin gene transcription via the protein kinase C pathway. Pflugers Arch. 2002 Jul;444(4):499–505. doi: 10.1007/s00424-002-0835-8. [DOI] [PubMed] [Google Scholar]
- 4.Seikaly MG, Arant BS, Jr., Seney FD., Jr. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–7. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004 Aug;44(2):223–9. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005 May 3;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, et al. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip goldblatt hypertensive rats. Hypertension. 2008 Jun;51(6):1590–6. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieto-Carrasquero MC, Botros FT, Kobori H, Navar LG. Collecting Duct Renin: A major player in Angiotensin II-dependent Hypertension. J Am Soc Hypertens. 2009 Mar;3(2):96–104. doi: 10.1016/j.jash.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prieto MC, Gonzalez AA, Navar LG. Evolving concepts on regulation and function of renin in distal nephron. Pflugers Arch. 2013 Jan;465(1):121–32. doi: 10.1007/s00424-012-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999 Dec;34(6):1265–74. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 11.Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, et al. Renin and kallikrein in connecting tubule of mouse. Kidney Int. 2003 Dec;64(6):2155–62. doi: 10.1046/j.1523-1755.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II Stimulates Renin in Inner Medullary Collecting Duct Cells via Protein Kinase C and Independent of Epithelial Sodium Channel and Mineralocorticoid Receptor Activity. Hypertension. 2011 Mar;57(3):594–9. doi: 10.1161/HYPERTENSIONAHA.110.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–35. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C, et al. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol Renal Physiol. 2010 Jan;298(1):F150–F157. doi: 10.1152/ajprenal.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen G, Contrepas A. Physiology and pharmacology of the (pro)renin receptor. Curr Opin Pharmacol. 2008 Apr;8(2):127–32. doi: 10.1016/j.coph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen G, Muller DN. The biology of the (pro)renin receptor. J Am Soc Nephrol. 2010 Jan;21(1):18–23. doi: 10.1681/ASN.2009030300. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002 Jun;109(11):1417–27. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int. 1996 Dec;50(6):1897–903. doi: 10.1038/ki.1996.511. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen G, Danser AH. The (pro)renin receptor: therapeutic consequences. Expert Opin Investig Drugs. 2006 Oct;15(10):1131–5. doi: 10.1517/13543784.15.10.1131. [DOI] [PubMed] [Google Scholar]
- 20.Navar LG, Harrison-Bernard LM. Intrarenal angiotensin II augmentation in angiotensin II dependent hypertension. Hypertens Res. 2000;23:291–301. doi: 10.1291/hypres.23.291. [DOI] [PubMed] [Google Scholar]
- 21.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive states. JRAAS. 2001 Mar;2:S176–S184. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–22. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiyama A, Seth DE, Navar LG. Renal interstitial concentrations of angiotensin I and angiotensin II in angiotensin II-infused hypertensive rats. Journal of the American Society of Nephrology. 2001;12:574A. Ref Type: Abstract. [Google Scholar]
- 24.Wang C-T, Navar LG, Mitchell KD. Proximal tubular fluid angiotensin II levels in angiotensin II-induced hypertensive rats. J Hypertens. 2003;21:353–60. doi: 10.1097/00004872-200302000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002 May;13(5):1131–5. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 26.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, et al. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003 Aug;42(2):195–9. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 27.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006 Nov 21;103(47):17985–90. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002 Feb;61(2):579–85. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003 Jan;41(1):42–9. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004 May;43(5):1126–32. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, et al. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol-Renal Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 32.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Urushihara M, Acres OW, et al. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol. 2009 Nov 13;311(1-2):24–31. doi: 10.1016/j.mce.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, et al. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol. 2008 Jul;295(1):F283–F289. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satou R, Miyata K, Gonzalez-Villalobos RA, Ingelfinger JR, Navar LG, Kobori H. Interferon-gamma biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells. FASEB J. 2012 May;26(5):1821–30. doi: 10.1096/fj.11-195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurtz A, Wagner C. Cellular control of renin secretion. Journal of Experimental Biology. 1999;202(3):219–25. doi: 10.1242/jeb.202.3.219. [DOI] [PubMed] [Google Scholar]
- 36.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol. 2009 May;296(5):F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao W, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension. 2010 Sep;56(3):378–83. doi: 10.1161/HYPERTENSIONAHA.110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M, Itoh H. The (pro)renin receptor and the kidney. Semin Nephrol. 2007 Sep;27(5):524–8. doi: 10.1016/j.semnephrol.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Ichihara A, Sakoda M, Kurauchi-Mito A, Kaneshiro Y, Itoh H. Involvement of (pro)renin receptor in the glomerular filtration barrier. J Mol Med (Berl) 2008 Jun;86(6):629–35. doi: 10.1007/s00109-008-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ichihara A. (Pro)renin receptor and autophagy in podocytes. Autophagy. 2012 Feb 1;8(2):271–2. doi: 10.4161/auto.8.2.18846. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble Form of the (Pro) Renin Receptor Is Augmented in the Collecting Duct and Urine of Chronic Angiotensin II-Dependent Hypertensive Rats. Hypertension. 2011 Apr;57(4):859–64. doi: 10.1161/HYPERTENSIONAHA.110.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, et al. (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes. 2009 Jul;58(7):1625–33. doi: 10.2337/db08-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deinum J, Ronn B, Mathiesen E, Derkx FH, Hop WC, Schalekamp MA. Increase in serum prorenin precedes onset of microalbuminuria in patients with insulin-dependent diabetes mellitus. Diabetologia. 1999 Aug;42(8):1006–10. doi: 10.1007/s001250051260. [DOI] [PubMed] [Google Scholar]
- 44.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, et al. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006 Aug;70(4):641–6. doi: 10.1038/sj.ki.5001627. [DOI] [PubMed] [Google Scholar]
- 45.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest. 2004 Oct;114(8):1128–35. doi: 10.1172/JCI21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008 Jun;51(6):1597–604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen G, Burckle CA, Sraer JD. Renin/prorenin-receptor biochemistry and functional significance. Curr Hypertens Rep. 2004 Apr;6(2):129–32. doi: 10.1007/s11906-004-0088-3. [DOI] [PubMed] [Google Scholar]
- 48.Prieto MC, Williams DE, Liu L, Kavanagh KL, Mullins JJ, Mitchell KD. Enhancement of renin and prorenin receptor in collecting duct of Cyp1a1-Ren2 rats may contribute to development and progression of malignant hypertension. Am J Physiol Renal Physiol. 2011 Feb;300(2):F581–F588. doi: 10.1152/ajprenal.00433.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009 Jun;53(6):1077–82. doi: 10.1161/HYPERTENSIONAHA.108.127258. [DOI] [PubMed] [Google Scholar]
- 50.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol-Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 51.Quinto BM, Pesquero JB, Casarini DE. Identification of a new site of angiotensin I-converting enzyme (ACE) production in IMCD cells and study of interaction between the bradykinin receptor B2 and ACE using ACE inhibitor. J Hypertens. 2002;20:S195. Ref Type: Abstract. [Google Scholar]
- 52.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-Independent Upregulation of Cyclooxygenase-2 by Activation of the (Pro)Renin Receptor in Rat Renal Inner Medullary Cells. Hypertension. 2013 Feb;61(2):443–9. doi: 10.1161/HYPERTENSIONAHA.112.196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vio CP, Cordova M, Alhenc-Gelas F. Increased angiotensin I-converting enzyme in epithelial, endothelial and interstitial cells in hypertensive kidneys. Hypertension. 1997;29:847. Ref Type: Abstract Do not cite abstracts. [Google Scholar]
- 54.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001 Mar;12:431–9. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen G, Burckle C, Sraer JD. The renin receptor: the facts, the promise and the hope. Curr Opin Nephrol Hypertens. 2003 Jan;12(1):51–5. doi: 10.1097/00041552-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, et al. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009 Aug;54(2):261–9. doi: 10.1161/HYPERTENSIONAHA.109.128645. [DOI] [PubMed] [Google Scholar]
- 57.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002 Sep;13(9):2207–12. doi: 10.1097/01.asn.0000026610.48842.cb. [DOI] [PubMed] [Google Scholar]
- 58.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–34. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 59.Zhao D, Navar LG. Acute angiotensin II infusions elicit pressure natriuresis in mice and reduce distal fractional sodium reabsorption. Hypertension. 2008 Jul;52(1):137–42. doi: 10.1161/HYPERTENSIONAHA.108.111435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension. 2009 Jul;54(1):120–6. doi: 10.1161/HYPERTENSIONAHA.109.133785. [DOI] [PMC free article] [PubMed] [Google Scholar]