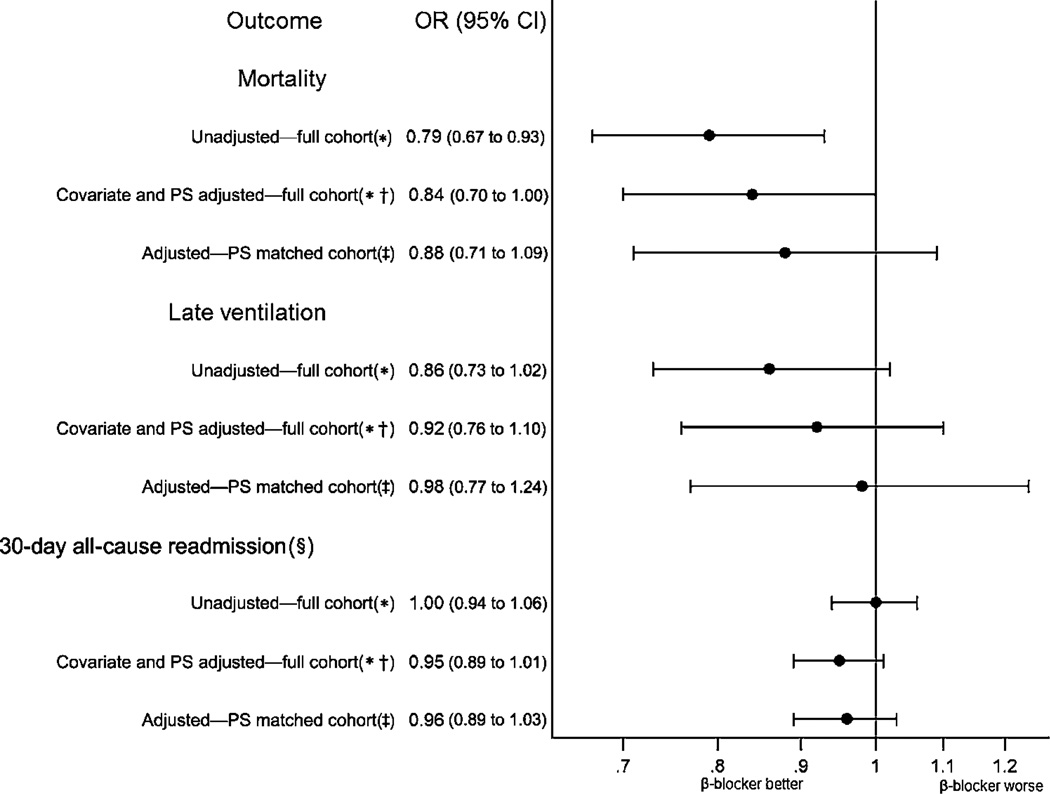
Figure 2. Outcomes of patients treated with a β blocker on day 1 or 2 compared with late treated or untreated patients. PS, propensity score.
*Accounting for within-hospital clustering. †Covariates included in all models: underlying cardiovascular condition (hypertension (HTN), ischaemic heart disease (IHD), congestive heart failure (CHF)), age group, gender, race/ethnicity status, insurance payor, prior year admissions for chronic obstructive pulmonary disease, principal diagnosis, attending physician specialty, atrial fibrillation/flutter, valvular disease, peripheral vascular disease, other neurological disease, diabetes, hypothyroidism, renal failure, liver disease, weight loss, lymphoma, metastatic cancer, solid tumour without metastasis, deficiency anaemias, drug abuse, obesity, psychoses, depression, polycythaemia, pneumonia, pulmonary embolism, pneumothorax, other infections; and day 1 or 2 aminoglycosides, carbapenems, anti-methicillin-resistant Staphylococcus aureus (MRSA) medications, third and fourth cephalosporins, third-generation penicillins, macrolides, long-acting anticholinergic bronchodilators, methylxanthine bronchodilators, steroids, arterial blood gas, non-invasive ventilation, brain natriuretic peptide test, morphine, chronic pulmonary heart disease, loop diuretics, ace inhibitors or angiotensin II receptor blockers, calcium channel blockers, digoxin, statins, lipid lowering non-statins, antiarrhythmics, warfarin, thiazide diuretics, nicotine cessation therapy; and hospital region, bed size, rural status and selected interaction terms. ‡Covariates included underlying cardiovascular condition (HTN, IHD, CHF), secondary International Classification of Disease code for prior MI, gender, previous year’s admissions for chronic obstructive pulmonary disease, attending physician specialty, atrial fibrillation/flutter, valvular disease, peripheral vascular disease, diabetes, renal failure, lymphoma, arthritis; and day 1 or 2 long-acting anticholinergic bronchodilators, methylxanthine bronchodilators, steroids, long-acting β2 agonists, arterial blood gas, non-invasive ventilation, chronic pulmonary heart disease, loop diuretics, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, nitrates, digoxin, antiplatelets, calcium channel blockers, aldosterone antagonists, statins, lipid-lowering non-statins, thiazide diuretics, warfarin; and hospital region; and selected interaction terms. §Among 34 265 survivors in the full cohort and 17 928 survivors in the matched cohort.