Abstract
Small molecular weight protein kinase inhibitors are frequently used tools to unravel the complex network of cellular signal transduction under certain physiological and pathophysiological conditions. 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine (PP2) is a widely used compound to block the activity of Src family kinases, the major group of non-receptor tyrosine kinases, which trigger multiple cellular signaling pathways. Here, we show that PP2 induces cytochrome P450 1A1 mRNA expression and enzyme activity in a dose-dependent manner in human HepG2 hepatoma cells and NCTC 2544 keratinocytes. By means of reporter gene assays, RNA interference, electrophoretic mobility shift assays and competitive ligand-binding assays, we further demonstrate that PP2 is a ligand for the aryl hydrocarbon receptor (AHR), an intracellular chemosensor that regulates xenobiotic metabolism, environmental stress responses and immune functions. Upon ligand-dependent activation, the AHR translocates into the nucleus and dimerizes with the AHR nuclear translocator (ARNT) to modulate the expression of its target genes. In addition, AHR activation is frequently accompanied by an activation of the tyrosine kinase c-Src, resulting in stimulation of cell-surface receptors and downstream signal transduction. As PP2 activates the AHR/ARNT pathway by simultaneously blocking c-Src-mediated alternative signaling routes, this compound may be a suitable tool to study the contribution of the different AHR-dependent signaling pathways to biological processes and adverse outcomes. On the other hand, the unexpected property of PP2 to stimulate AHR/ARNT signaling should be taken into account carefully in future investigations in order to avoid a false interpretation of experimental results and molecular interrelations.
Introduction
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor of the basic Helix-Loop-Helix PAS protein family, whose members regulate gene expression in response to developmental and environmental signals [1]. In absence of a ligand, the AHR rests in a cytosolic multiprotein complex consisting of heat-shock protein 90, AHR-interacting protein, and co-chaperone p23. Upon ligand-binding, the AHR sheds its chaperones, translocates in the nucleus, heterodimerizes with the AHR nuclear translocator (ARNT) and binds to xenobiotic-responsive elements (XRE) in the promoter region of target genes to induce their expression. The AHR gene battery encodes for xenobiotic-metabolizing enzymes, such as cytochrome P450 (CYP) 1A1, as well as for proteins involved in regulation of proliferation, differentiation and apoptosis [2].
The AHR is activated by a broad spectrum of environmental pollutants, including dioxins and polycyclic aromatic hydrocarbons (PAHs) [2; 3] and gene targeting studies in rodents have identified the AHR as an essential mediator of dioxin toxicity and PAH carcinogenicity [4–7]. In addition, the AHR in keratinocytes is activated upon solar ultraviolet (UV) B irradiation to initiate parts of the cellular UVB stress response, e.g. induction of pro-inflammatory cyclooxygenase-2 (COX-2) [8]. The initial event for this UVB-mediated AHR activation is the absorbance of UVB rays by free tryptophan followed by the intracellular formation of the tryptophan photoproduct 6-formylindolo[3,2b]carbazole, a high-affinity AHR ligand [8; 9]. Mechanistic studies aiming to elucidate the complex processes triggering both dioxin toxicity and cutaneous UVB stress responses have brought to light that the AHR signals not only in an ARNT-dependent manner, but also through an alternative pathway [10; 11]. Activation of the AHR leads to a rapid activation of the soluble tyrosine kinase c-Src, which is either directly or indirectly associated with the cytosolic AHR multiprotein complex [12; 13]. Activated c-Src can translocate to the plasma membrane and phosphorylate cell-surface receptors, such as the epidermal growth factor receptor (EGFR), which leads to the subsequent stimulation of downstream mitogen-activated protein kinase (MAPK) signal transduction to modulate transcription of genes, such as COX-2 [8; 14].
As c-Src is the molecular link between ligand-mediated AHR activation and accompanied EGFR phosphorylation, chemical inhibitors of this tyrosine kinase are frequently used to study an involvement of alternative AHR signaling in certain cellular processes [8; 15–17]. One of the most prominent compounds used to inhibit c-Src and related Src family kinases (SFKs) is 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine, also known as PP2 (Figure 1A) [18]. SFK activity is regulated by phosphorylation and dephosphorylation of a certain tyrosine residue (e.g. Y530 for c-Src), which is triggered by C-terminal Src kinase and protein tyrosine phosphatases [19]. Phosphorylation of this tyrosine residue results in an inactivation of the SFK, whereas its dephosphorylation leads to a destabilization of intramolecular interactions and a subsequent autophosphorylation of another tyrosine residue (e.g. Y416 for c-Src). This ATP-dependent autophosphorylation triggers conformational opening of the SFK molecule and enables an interaction with receptor tyrosine kinases, cytokine receptors, G-protein-coupled receptors and other signaling molecules [19]. PP2 now binds to the ATP-binding site in the catalytic center of the SFK and thereby inhibits the kinase-activating process of autophosphorylation [20].
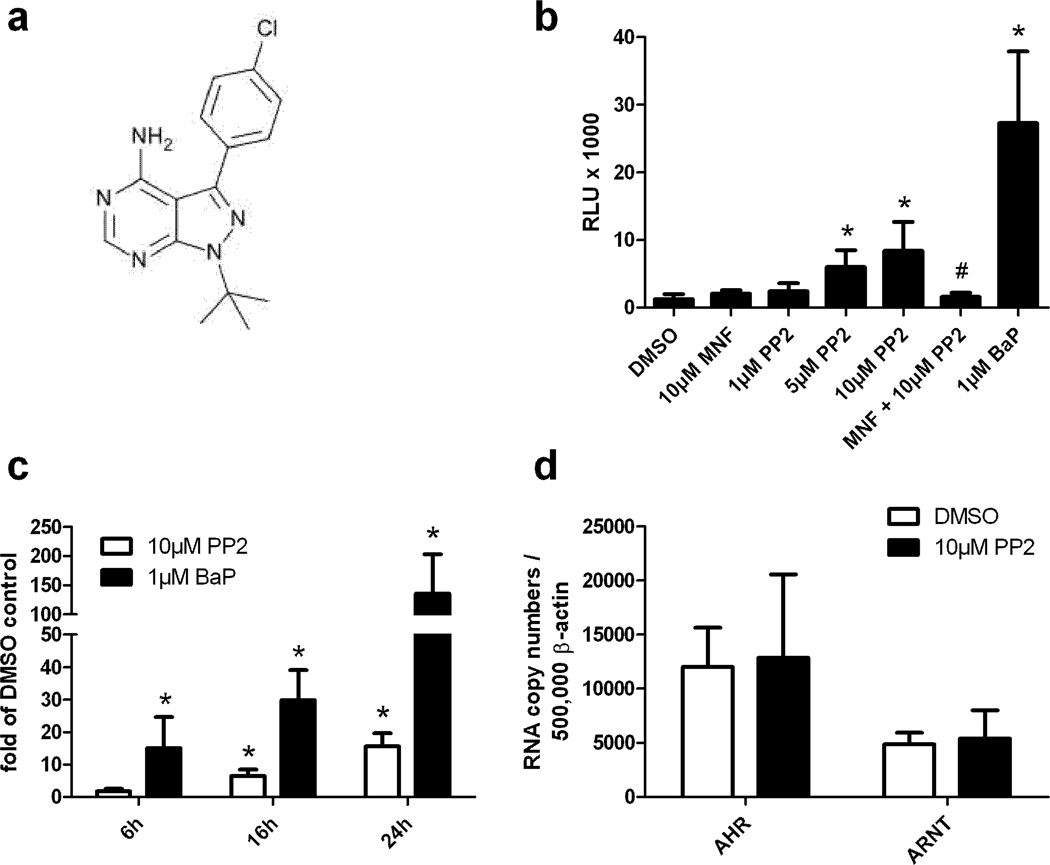
Figure 1. PP2 induces XRE-dependent reporter gene activity and CYP1A1 mRNA expression in HepG2 cells.
A. Structure of PP2. B. XRE-HepG2 cells were treated with PP2 (1µM, 5µM, 10µM), 10µM MNF, 10µM MNF plus 10µM PP2, 1µM BaP or solvent. After 24h luciferase activities were determined and normalized to protein content. Results are shown as relative light units (RLU). *, significantly increased compared to solvent-treated samples, #, significantly decreased compared to 10µM PP2-treated samples. C. HepG2 cells were exposed for 8h, 16h, and 24h to 10µM PP2 or solvent. CYP1A1 and β-actin gene expression was analyzed by quantitative real-time PCR. Results are shown as fold of solvent ctrl. *, significantly increased compared to solvent-treated samples. D. HepG2 cells were treated with 10µM PP2 or solvent. After 24h cells were harvested and gene expression of AHR, ARNT, and β-actin was analyzed by quantitative real-time PCR.
In the current study, we identify activation of AHR signaling as an off-target effect of PP2 in human immortalized cell-lines and further disclose this compound as a true ligand of AHR.
Results and Discussion
While studying the role of alternative AHR signaling in UVB-irradiated keratinocytes using different protein kinase inhibitors, we unexpectedly observed a transcriptional induction of CYP1A1 in PP2-treated, sham-irradiated cells. To clarify if PP2 activates canonical AHR signaling, we performed reporter gene assays in XRE-HepG2 cells. These cells contain a stable integrated luciferase reporter construct driven by two XREs [21]. A 24h exposure of XRE-HepG2 cells to different concentrations of PP2 resulted in a dose-dependent increase in luciferase activity thereby confirming that PP2 stimulates AHR-dependent transcription (Figure 1B). Whereas treatment of the cells with 1µM of the PAH and model AHR agonist benzo(a)pyrene (BaP) resulted in a 21-fold induction of luciferase activity, an exposure to 5µM and 10µM PP2 led to a 4.5-fold and 6.5-fold increase of the response, respectively. To prove the AHR-dependency of the PP2-mediated induction of luciferase activity, we treated XRE-HepG2 cells for 1h with 3’-methoxy-4’-nitroflavone (MNF), an established AHR antagonist [22], prior to PP2 exposure. As expected, the reporter gene activity of cells exposed to 10µM MNF and 10µM PP2 was significantly reduced compared to the cells treated solely with PP2 (Figure 1B). Thus, it is highly likely that the PP2-induced increase in promoter activity is due to a direct binding of PP2 to the AHR protein. To confirm these data, we treated HepG2 cells with 10µM PP2 or solvent and measured gene expression of the AHR prototype target gene CYP1A1 by quantitative real-time PCR. PP2 exposure resulted in a time-dependent transcriptional up-regulation of CYP1A1. After 24h, CYP1A1 mRNA was approximately 15-fold elevated by PP2, whereas an exposure to 1µM BaP led to a roughly 150-fold induction (Figure 1C). To exclude the possibility that PP2 increased the expression level of AHR or ARNT and thereby just indirectly raised XRE-dependent gene expression, we compared the expression of both genes in solvent- and PP2-treated HepG2 cells. Notably, an exposure for 24h to 10µM PP2 had no effect on AHR and ARNT mRNA expression (Figure 1D).
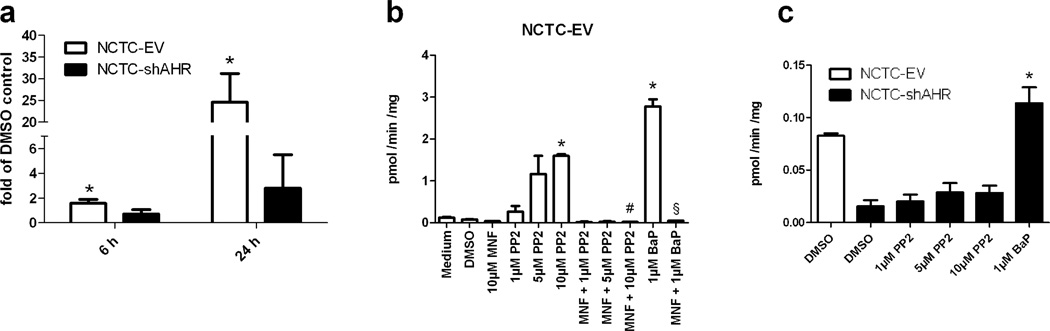
Next, we investigated the effect of PP2 treatment on CYP1A1 transcription in AHR-proficient and AHR-knockdown keratinocytes. More precisely, we used human immortalized NCTC 2544 keratinocytes, which were stable transfected with an AHR-targeting shRNA-construct (NCTC-shAHR) or the respective empty vector (NCTC-EV), as described previously [23]. Exposure of NCTC-EV cells to 10µM PP2 for 6h resulted in a slight but significant induction of CYP1A1 transcription (Figure 2A). After 24h of treatment the message was approximately 25-fold enhanced. As expected, PP2 treatment of NCTC-shAHR cells did not alter CYP1A1 transcription (Figure 2A), demonstrating that PP2 acts exclusively in an AHR-dependent manner. To ensure that the observed alterations on transcription are translated to functional level, we performed 7-O-ethoxyresorufin-deethylase (EROD) assays to measure CYP1A enzyme activity. An exposure to increasing concentrations of PP2 (1µM, 5µM, 10µM) for 24h significantly induced EROD activity in NCTC-EV cells in a dose-dependent manner (Figure 2B). In comparison to solvent-treated cells, EROD activity was 10-fold enhanced by 10µM PP2, whereas 1µM BaP exposure elevated EROD activity roughly 20-fold. A 1h pre-treatment with 10µM MNF completely abolished PP2- and BaP-induced CYP1A enzyme activities. Accordingly, PP2 treatment did not affect CYP1A activity in NCTC-shAHR cells, but notably the residual AHR level in these cells [approximately 20% [23]] was still sufficient to marginally induce EROD activity in response to BaP exposure (Figure 2C). These results confirm the observed PP2-mediated and AHR-dependent increase in CYP1A1 transcription and indicate that PP2 usage may potentially influence experimental results by modulating the CYP1A-driven metabolism of endogenous substrates and co-administered chemicals and drugs. In addition, the PP2-activated AHR may also modulate the expression of genes encoding for proteins regulating other cellular functions, or it may affect the activity of interacting transcription factors (e.g. NF-κB, hypoxia-inducible factors, estrogen receptors) [2; 3].
Figure 2. Effect of PP2 treatment on CYP1A1 mRNA expression and CYP1A enzyme activity in NCTC-EV and NCTC-shAHR keratinocytes.
A. NCTC-EV and NCTC-shAHR cells were treated for 6h and 24h with 10µM PP2 or solvent. Transcription of CYP1A1 and β-actin was analyzed by quantitative real-time PCR. Results are shown as fold of solvent ctrl. *, significantly increased compared to solvent-treated samples. B. NCTC-EV cells were treated with PP2 (1µM, 5µM, 10µM), 10µM MNF, 10µM MNF plus 10µM PP2, 1µM BaP or solvent. After 24h EROD activities were measured. *, significantly increased compared to solvent-treated samples; #, significantly reduced compared to 10µM PP2-treated samples, §, significantly reduced compared to BaP-treated samples. C. NCTC-shAHR cells were treated with PP2 (1µM, 5µM, 10µM), 1µM BaP or solvent and EROD activities were measured after 24h. *, significantly reduced compared to solvent-treated NCTC-EV cells.
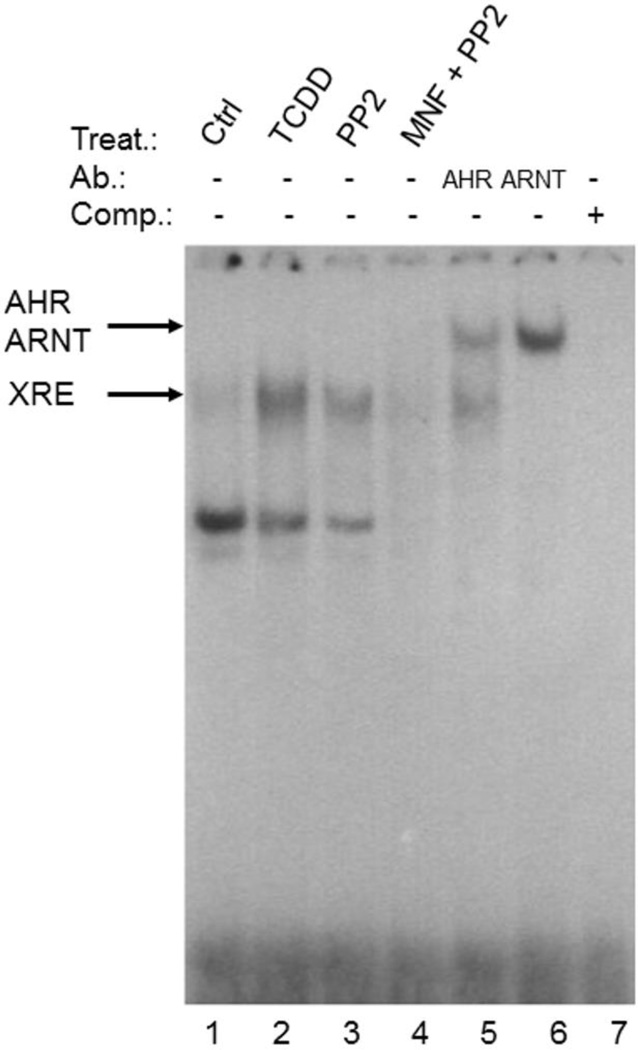
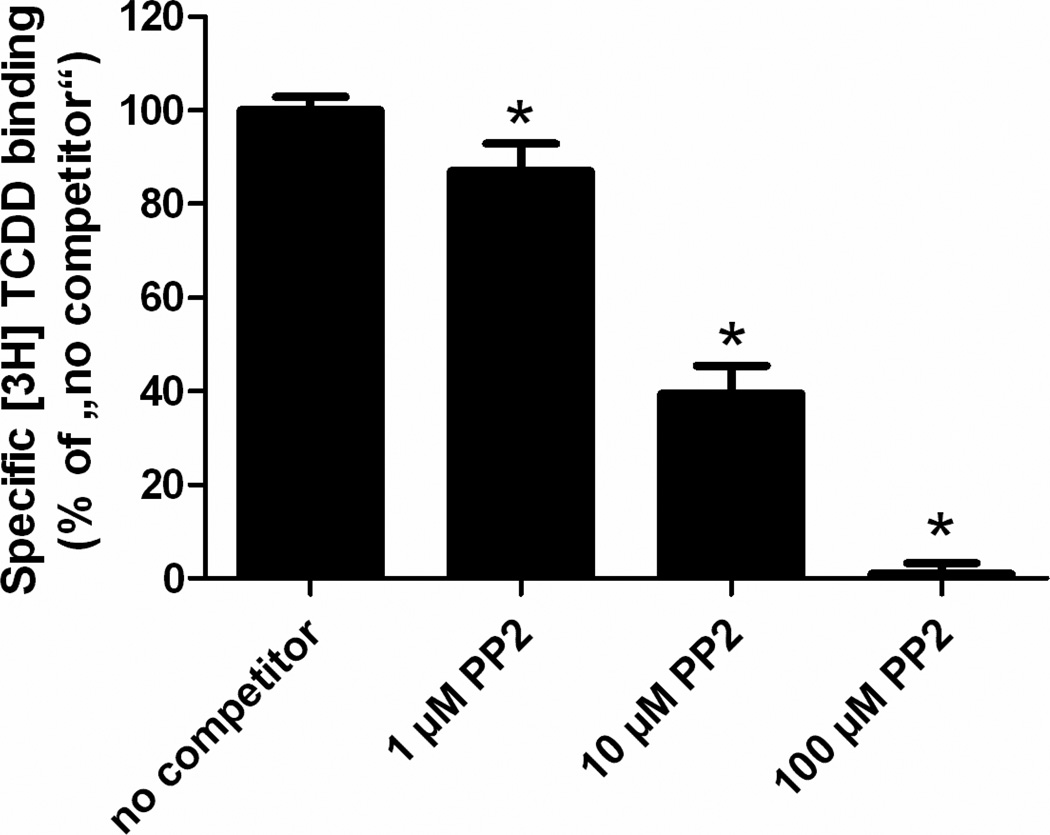
To ensure that PP2 stimulates the DNA-binding activity of AHR/ARNT, we performed electrophoretic mobility shift assays (EMSA) using a radioactive-labeled XRE-containing oligonucleotide. Treatment of HepG2 cells with 1nM of the prototype AHR ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) resulted in a strong band shift, representing the XRE-bound AHR/ARNT complex (Figure 3). Upon exposure of the cells to 10µM PP2 we observed a clear band shift of lower intensity compared to TCDD-treated samples, which completely disappeared after co-treating the cells with 10µM MNF. The addition of antibodies targeting either AHR or ARNT to the nuclear extracts of PP2-treated HepG2 cells led to a supershift of the band, providing evidence that both proteins were bound to the XRE-bearing oligonucleotide. Thus, PP2 activates AHR and initiates the formation of a nuclear AHR/ARNT heterodimer that recognizes its DNA target motif to modulate gene expression. Notably, the lower band observed in the EMSA may represent a binding complex of AHR containing other partner proteins (i.e. Rel proteins), as described earlier [24]. To assess if PP2 activates AHR in a ligand-dependent manner, we performed competitive ligand-binding assays using guinea pig hepatic cytosol. As shown in figure 4, the addition of 1µM and 10µM PP2 reduced the specific binding of [3H]TCDD to AHR protein to 87% and 40%, respectively. Notably, the co-incubation with 100µM PP2 resulted in a complete displacement of [3H]TCDD from AHR. These dose-dependent effects of PP2 identify this SFK inhibitor as a ligand of AHR.
Figure 3. PP2 stimulates XRE-binding activity of a nuclear AHR/ARNT heterodimer in HepG2 cells.
HepG2 cells were treated with 1nM TCDD, 10µM PP2 and 10µM PP2 plus 10µM MNF for 2h prior to isolation of nuclear extracts and were hybridized with a XRE consensus oligonucleotide. Supershift analyses were done by adding 2µg of either anti-AHR or anti-ARNT to nuclear extracts of PP2-treated cells. A representative EMSA of two independent experiments is shown.
Figure 4. The SFK inhibitor PP2 is a ligand of the AHR.
Untreated guinea pig hepatic cytosol was incubated with 2 nM [3H]-TCDD alone (total binding), 2nM 3HTCDD and 200nM TCDF (non-specific binding) or 2nM 3HTCDD in the presence of 1µM, 10µM or 100µM PP2. Samples were analyzed by the hydroxyapatite assay as described under Materials and Methods. Specific binding was determined as a difference between total and non-specific binding reactions. The values are presented as mean (± standard deviation) of three independent reactions. *, significantly different from the ‘no competitor’ reaction. Representative results of two independent experiments are shown.
SFKs are the major group of non-receptor tyrosine kinases and its members play crucial roles in signal transduction and thus are of fundamental importance for numerous biological functions, including proliferation, differentiation, adhesion, migration, apoptosis, autophagy and angiogenesis [19]. c-Src was the first SFK discovered as the cellular form of the transforming gene product v-Src of the avian Rous sarcoma virus [25]. Meanwhile, ten SFKs were identified, differing in their cellular distribution pattern: Whereas c-Src, Fyn and Yes are ubiquitously expressed, the expression of Blk, Fgr, Hck, Lck, Yrk and Lyn is restricted to hematopoietic cells, and that of Frk to cells of epithelial origin [19]. Multiple studies provided evidence that c-Src and other SFKs are involved in the development, progression and metastasis of several human malignancies, including prostate, breast, colon, head and neck, lung and pancreatic cancer [19; 26; 27]. Accordingly, the elucidation of the functional roles of SFKs under physiological and pathophysiological conditions is of high priority. Hence, SFK inhibitors, such as PP2, are frequently used tools to dissect SFK function. As the AHR and its binding partner ARNT are expressed in nearly all normal and malignant cell-types and tissues [2; 28], it cannot be excluded that some of the experimental observations made in PP2-exposed cells or tissues were not due to PP2-mediated SFK inhibition, but to a PP2-initiated activation of canonical AHR signaling. Moreover, yet unexplained experimental observations, such as the PP2-mediated enhancement of lipopolysaccharide-induced IL-6 expression in macrophages [29], may be explainable by a PP2-driven activation of AHR signaling. Indeed, a comparable synergistic induction of IL-6 was recently identified in MCF-7 cells co-exposed to TCDD and NF-κB-stimulatory IL-1β, and probably involved a cross-talk between AHR and RelA [30]. On the other hand, the property to activate canonical AHR signaling by contemporaneously inhibiting c-Src-dependent alternative AHR pathways, may suit PP2 as a useful tool for mechanistic studies on AHR biology.
The vast majority of chemicals designed to interfere with protein kinase activity act through binding to the ATP-binding site of the respective enzyme [31]. Accordingly, putative kinase inhibitors are limited in their size and structural diversity, implying that other kinase blockers may also interact with AHR. In fact, an increasing number of protein kinase inhibitors, frequently used for research purposes [e.g. the MEK inhibitor PD98059 [32], the p38 MAPK inhibitor SB203580 [33], and others] or in cancer chemotherapy [e.g. the broadband receptor tyrosine kinase inhibitor sunitinib [34] and the vascular endothelial growth factor receptor inhibitor semaxinib [35]], were meanwhile identified to manipulate AHR activity. Beside the mentioned danger of misinterpreting experimental data, an unpredicted modulation of AHR activity by clinically applied protein kinase inhibitors may contribute to the development of toxic side-effects (CYP1-mediated drug-drug interactions, adverse skin reactions, alterations of immune and endocrine functions) that may threaten patients’ health. Therefore, the potential of small molecular weight protein kinase inhibitors to manipulate AHR activity and downstream signaling events should be taken into account carefully in future research investigations and drug safety assessments.
Material and Methods
Cell Culture and Treatment
HepG2 cells and XRE-HepG2 reporter cells were cultured in RPMI-1640 medium (PAA, Coelbe, Germany) containing 3.7% NaHCO310% FCS and 1% antibiotic/antimycotic mixture. Culture medium for XRE-HepG2 cells was supplemented with 0.8mg/ml G418 (AppliChem, Darmstadt, Germany). NCTC-EV and NCTC-shAHR cells were cultured in MEM medium (PAA) supplemented with 10% FCS, 1% antibiotic/antimycotic mixture and 0.84mg/ml G418. All cells were cultivated in a humidified atmosphere of 5% CO2 at 37°C. PP2, BaP (Sigma-Aldrich, Munich, Germany), and MNF were dissolved in DMSO. For AHR antagonism, cells were 1h pretreated with MNF before PP2 or BaP were added as indicated.
Reporter gene assay
For reporter gene analyses, 1×105 XRE-HepG2 cells/well were seeded in 12-well plates and cultured overnight. Cells were treated as indicated and 24h later cell lysates were prepared and luciferase activities were determined using the luciferase assay system (Promega, Mannheim, Germany) in a Multi-Bioluminat LB 9505C (Berthold, Wildbad, Germany). Protein concentrations were determined by using the BC Assay protein quantification kit (BioRad). Luciferase activities were normalized against protein concentration.
Quantitative Real-Time PCR
For quantitative gene expression analyses, 3×105 cells/well were seeded into six-well plates, cultured overnight and treated at a confluence of approximately 70% as indicated. Total RNA was isolated using the Gold RNA kit (Peqlab, Erlangen, Germany). For each sample 0.5 µg of total RNA was reverse transcribed using MMLV reverse transcriptase (Invitrogen, Karlsruhe, Germany) in a total volume of 20 µl. Three microliters of cDNA of a 1:3 dilution were used for real-time PCR with SYBR Fast Reagent (Qiagen, Hilden, Germany) in a Corbett-Rotor Gene 300 light cycler (Qiagen). Gene expression was normalized to β-actin. The sequences of the oligonucleotides used were published previously [36].
O-Ethoxyresorufin-Deethylase (EROD) Assay
For measuring CYP1A1 activities in living monolayer keratinocyte cultures, ethoxyresorufin (Sigma-Aldrich; solved in DMSO) was employed according to a protocol described by [37]. Resorufin as the reaction product in the respective media or assay solution was used to generate standard curves. Shortly, serum-free media containing 2.5µM ethoxyresorufin and 10µM dicumarol were applied to PBS-washed monolayer cells and resorufin formation kinetics were measured 21 min at 37°C at excitation and emission wavelength of 544 nm and 590 nm on a Fluoroskan Ascent reader (Labsystems, Bornheim, Germany). Cells were treated for 24h with PP2, BaP and/or MNF as indicated. All experiments were carried out three times in triplicate.
Electrophoretic Mobility Shift Assay (EMSA)
Isolation of nuclear extracts and EMSAs were carried out as described previously [24]. The antibodies against AHR and ARNT used for supershift analyses were purchased from Novus Biologicals (Littleton, CO) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively.
AHR Ligand-binding Assay
The competitive displacement of 3HTCDD (specific activity 10.7 Ci/mmol) from guinea pig hepatic cytosol was performed as described earlier [33; 38]. Briefly, guinea pig cytosol was diluted to 8mg protein/ml with MEDG (25mM MOPS, pH 7.5, 1mM EDTA, 1mM DTT, 10% (v/v) glycerol) and incubated for 1h at room temperature with increasing concentrations of PP2 (1, 10, 100µM) or 200nM tetrachlorodibenzofuran (TCDF) in the presence of 2nM 3HTCDD. Next, reactions were incubated with 250µl of hydroxyapatite suspension for additional 30 min. Thereafter, reactions were washed three times with 1ml MEGT buffer (25mM MOPS, pH 7.5, 1mM EDTA, 10% (v/v) glycerol, 0.5% (v/v) Tween 80). The pellets were transferred into scintillation vials and counted in a scintillation counter. Specific binding was determined as the difference between the ‘no competitor’ and TCDF reaction.
Statistical Analyses
If not otherwise indicated, all data shown are mean (± standard deviation) of three or more independent experiments. Data were analyzed using two-sided Student’s t-test. P-values below 0.05 were considered as significant.
Acknowledgement
We thank Katarina Gradin and Lorenz Poellinger (Karolinska Institutet, Sweden) for providing the XRE-HepG2 cell-line. TCDF and [3H]TCDD were kindly provided by Stephen Safe (Texas A&M University, TX). The MNF was a gift from Gabriele Vielhaber and Imke Meyer (Symrise, Holzminden, Germany). We thank Kerstin Fischer, Birgit Neumann and Diane Schmiegelt for excellent technical support. Funding was provided to MSD by the National Institutes of Environmental Health Sciences (R01ES07685).
References
- 1.Gu YZ, Hogenesch JB, Bradfield CA. The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 2000;40:519–561. doi: 10.1146/annurev.pharmtox.40.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol. Chem. 2010;391:1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- 3.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- 6.Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 8.Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, Klotz LO, Rannug A, Furst P, Hanenberg H, Abel J, Krutmann J. Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rannug U, Rannug A, Sjoberg U, Li H, Westerholm R, Bergman J. Structure elucidation of two tryptophan-derived, high affinity Ah receptor ligands. Chem. Biol. 1995;2:841–845. doi: 10.1016/1074-5521(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura F. The significance of the nongenomic pathway in mediating inflammatory signaling of the dioxin-activated Ah receptor to cause toxic effects. Biochem. Pharmacol. 2009;77:608–626. doi: 10.1016/j.bcp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Haarmann-Stemmann T, Bothe H, Abel J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem. Pharmacol. 2009;77:508–520. doi: 10.1016/j.bcp.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Dong B, Cheng W, Li W, Zheng J, Wu D, Matsumura F, Vogel CF. FRET analysis of protein tyrosine kinase c-Src activation mediated via aryl hydrocarbon receptor. Biochim. Biophys. Acta. 2011;1810:427–431. doi: 10.1016/j.bbagen.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S, Dong B, Matsumura F. Rapid activation of c-Src kinase by dioxin is mediated by the Cdc37-HSP90 complex as part of Ah receptor signaling in MCF10A cells. Biochemistry. 2007;46:899–908. doi: 10.1021/bi061925f. [DOI] [PubMed] [Google Scholar]
- 14.Vogel C, Boerboom AM, Baechle C, El-Bahay C, Kahl R, Degen GH, Abel J. Regulation of prostaglandin endoperoxide H synthase-2 induction by dioxin in rat hepatocytes: possible c-Src-mediated pathway. Carcinogenesis. 2000;21:2267–2274. doi: 10.1093/carcin/21.12.2267. [DOI] [PubMed] [Google Scholar]
- 15.Xie G, Peng Z, Raufman JP. Src-mediated aryl hydrocarbon and epidermal growth factor receptor cross talk stimulates colon cancer cell proliferation. Am. J. Physiol Gastrointest. Liver Physiol. 2012;302:G1006–G1015. doi: 10.1152/ajpgi.00427.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomkiewicz C, Herry L, Bui LC, Metayer C, Bourdeloux M, Barouki R, Coumoul X. The aryl hydrocarbon receptor regulates focal adhesion sites through a non-genomic FAK/Src pathway. Oncogene. 2013;32:1811–1820. doi: 10.1038/onc.2012.197. [DOI] [PubMed] [Google Scholar]
- 17.Randi AS, Sanchez MS, Alvarez L, Cardozo J, Pontillo C, Kleiman de Pisarev DL. Hexachlorobenzene triggers AhR translocation to the nucleus, c-Src activation and EGFR transactivation in rat liver. Toxicol. Lett. 2008;177:116–122. doi: 10.1016/j.toxlet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a Novel, Potent, and Src Family-selective Tyrosine Kinase Inhibitor: Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14:667–678. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karni R, Mizrachi S, Reiss-Sklan E, Gazit A, Livnah O, Levitzki A. The pp60c-Src inhibitor PP1 is non-competitive against ATP. FEBS Lett. 2003;537:47–52. doi: 10.1016/s0014-5793(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 21.Haarmann-Stemmann T, Sendker J, Gotz C, Krug N, Bothe H, Fritsche E, Proksch P, Abel J. Regulation of dioxin receptor function by different beta-carboline alkaloids. Arch. Toxicol. 2010;84:619–629. doi: 10.1007/s00204-010-0548-2. [DOI] [PubMed] [Google Scholar]
- 22.Lu YF, Santostefano M, Cunningham BD, Threadgill MD, Safe S. Identification of 3'-methoxy-4'-nitroflavone as a pure aryl hydrocarbon (Ah) receptor antagonist and evidence for more than one form of the nuclear Ah receptor in MCF-7 human breast cancer cells. Arch. Biochem. Biophys. 1995;316:470–477. doi: 10.1006/abbi.1995.1062. [DOI] [PubMed] [Google Scholar]
- 23.Frauenstein K, Sydlik U, Tigges J, Majora M, Wiek C, Hanenberg H, Abel J, Esser C, Fritsche E, Krutmann J, Haarmann-Stemmann T. Evidence for a novel anti-apoptotic pathway in human keratinocytes involving the aryl hydrocarbon receptor, E2F1, and checkpoint kinase 1. Cell Death. Differ. 2013;20:1425–1434. doi: 10.1038/cdd.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim. Biophys. Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 26.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 27.Saito YD, Jensen AR, Salgia R, Posadas EM. Fyn: a novel molecular target in cancer. Cancer. 2010;116:1629–1637. doi: 10.1002/cncr.24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng S, Cao Z, Wang X. Role of aryl hydrocarbon receptor in cancer. Biochim. Biophys. Acta. 2013;1836:197–210. doi: 10.1016/j.bbcan.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen TT, Johnsen IB, Knetter CF, Drablos F, Fitzgerald KA, Lien E, Anthonsen MW. Differential gene expression downstream of Toll-like receptors (TLRs): role of c-Src and activating transcription factor 3 (ATF3) J. Biol. Chem. 2010;285:17011–17019. doi: 10.1074/jbc.M109.068817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiNatale BC, Schroeder JC, Francey LJ, Kusnadi A, Perdew GH. Mechanistic insights into the events that lead to synergistic induction of interleukin 6 transcription upon activation of the aryl hydrocarbon receptor and inflammatory signaling. J. Biol. Chem. 2010;285:24388–24397. doi: 10.1074/jbc.M110.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiners JJ, Jr, Lee JY, Clift RE, Dudley DT, Myrand SP. PD98059 is an equipotent antagonist of the aryl hydrocarbon receptor and inhibitor of mitogen-activated protein kinase kinase. Mol. Pharmacol. 1998;53:438–445. doi: 10.1124/mol.53.3.438. [DOI] [PubMed] [Google Scholar]
- 33.Korashy HM, Anwar-Mohamed A, Soshilov AA, Denison MS, El-Kadi AO. The p38 MAPK inhibitor SB203580 induces cytochrome P450 1A1 gene expression in murine and human hepatoma cell lines through ligand-dependent aryl hydrocarbon receptor activation. Chem. Res. Toxicol. 2011;24:1540–1548. doi: 10.1021/tx200141p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maayah ZH, El Gendy MA, El-Kadi AO, Korashy HM. Sunitinib, a tyrosine kinase inhibitor, induces cytochrome P450 1A1 gene in human breast cancer MCF7 cells through ligand-independent aryl hydrocarbon receptor activation. Arch. Toxicol. 2013;87:847–856. doi: 10.1007/s00204-012-0996-y. [DOI] [PubMed] [Google Scholar]
- 35.Mezrich JD, Nguyen LP, Kennedy G, Nukaya M, Fechner JH, Zhang X, Xing Y, Bradfield CA. SU5416, a VEGF receptor inhibitor and ligand of the AHR, represents a new alternative for immunomodulation. PLoS One. 2012;7:e44547. doi: 10.1371/journal.pone.0044547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tigges J, Weighardt H, Wolff S, Gotz C, Forster I, Kohne Z, Huebenthal U, Merk HF, Abel J, Haarmann-Stemmann T, Krutmann J, Fritsche E. Aryl hydrocarbon receptor repressor (AhRR) function revisited: repression of CYP1 activity in human skin fibroblasts is not related to AhRR expression. J. Invest Dermatol. 2013;133:87–96. doi: 10.1038/jid.2012.259. [DOI] [PubMed] [Google Scholar]
- 37.Rolsted K, Kissmeyer AM, Rist GM, Hansen SH. Evaluation of cytochrome P450 activity in vitro, using dermal and hepatic microsomes from four species and two keratinocyte cell lines in culture. Arch. Dermatol. Res. 2008;300:11–18. doi: 10.1007/s00403-007-0811-4. [DOI] [PubMed] [Google Scholar]
- 38.Denison MS, Harper PA, Okey AB. Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Codistribution of unoccupied receptor with cytosolic marker enzymes during fractionation of mouse liver, rat liver and cultured Hepa-1c1 cells. Eur. J. Biochem. 1986;155:223–229. doi: 10.1111/j.1432-1033.1986.tb09480.x. [DOI] [PubMed] [Google Scholar]