Fig 7. Resolved 180s loop.
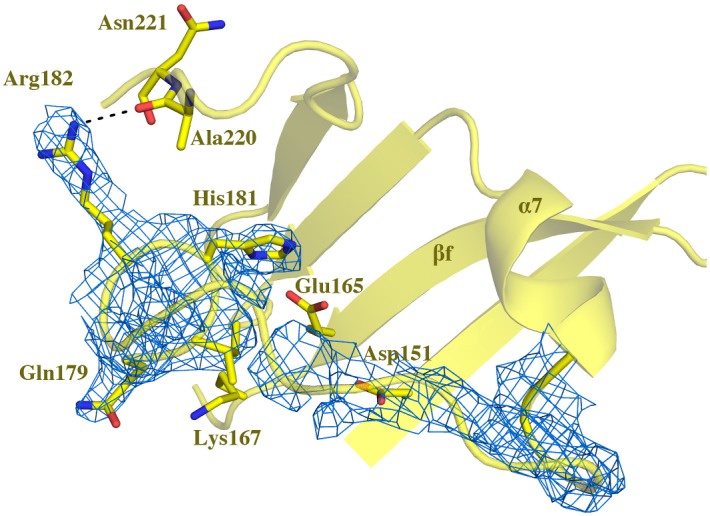
Electron density (blue mesh) in Lys220Ala mutant HsdR is shown only for the 180s loop, with selected sidechains of the loop shown as sticks in atomic colors with yellow carbons. Outside the 180s loop the sidechains of residues Asp151, Glu165, and Lys167 in the active site, and of Ala220 and Asn221 in the 220s loop, are labeled and shown as sticks, and alpha helix 7 and beta strand f are labeled. The dashed line indicates a distance short enough to permit bonding between the indicated functional groups. Coloring as in Fig 6.
