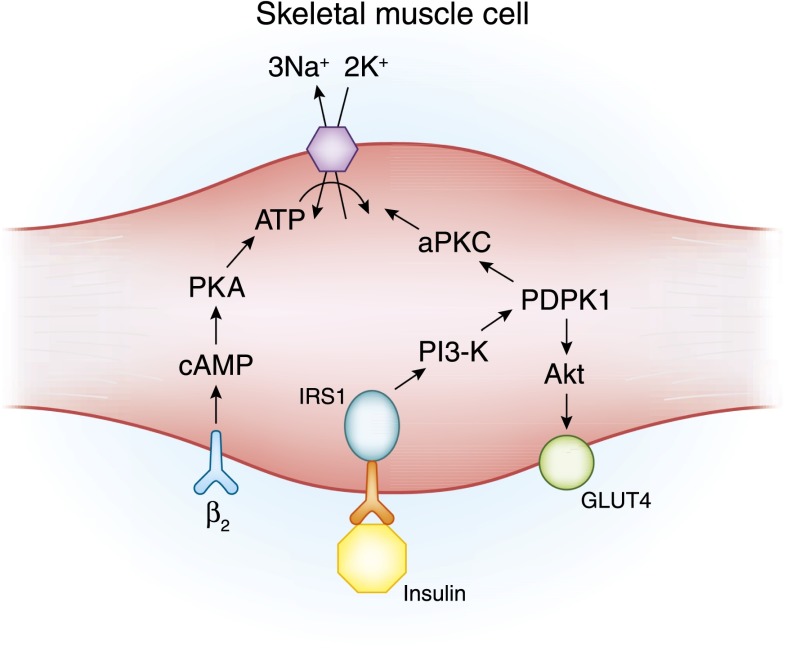
Figure 1.
The cell model illustrates β2-adrenergic and insulin-mediated regulatory pathways for K+ uptake. β2-Adrenergic and insulin both lead to K+ uptake by stimulating the activity of the Na+-K+-ATPase pump primarily in skeletal muscle, but they do so through different signaling pathways. β2-Adrenergic stimulation leads to increased pump activity through a cAMP- and protein kinase A (PKA)–dependent pathway. Insulin binding to its receptor leads to phosphorylation of the insulin receptor substrate protein (IRS-1), which, in turn, binds to phosphatidylinositide 3-kinase (PI3-K). The IRS-1–PI3-K interaction leads to activation of 3-phosphoinositide–dependent protein kinase-1 (PDK1). The stimulatory effect of insulin on glucose uptake and K+ uptake diverge at this point. An Akt-dependent pathway is responsible for membrane insertion of the glucose transporter GLUT4, whereas activation of atypical protein kinase C (aPKC) leads to membrane insertion of the Na+-K+-ATPase pump (reviewed in ref. 3).