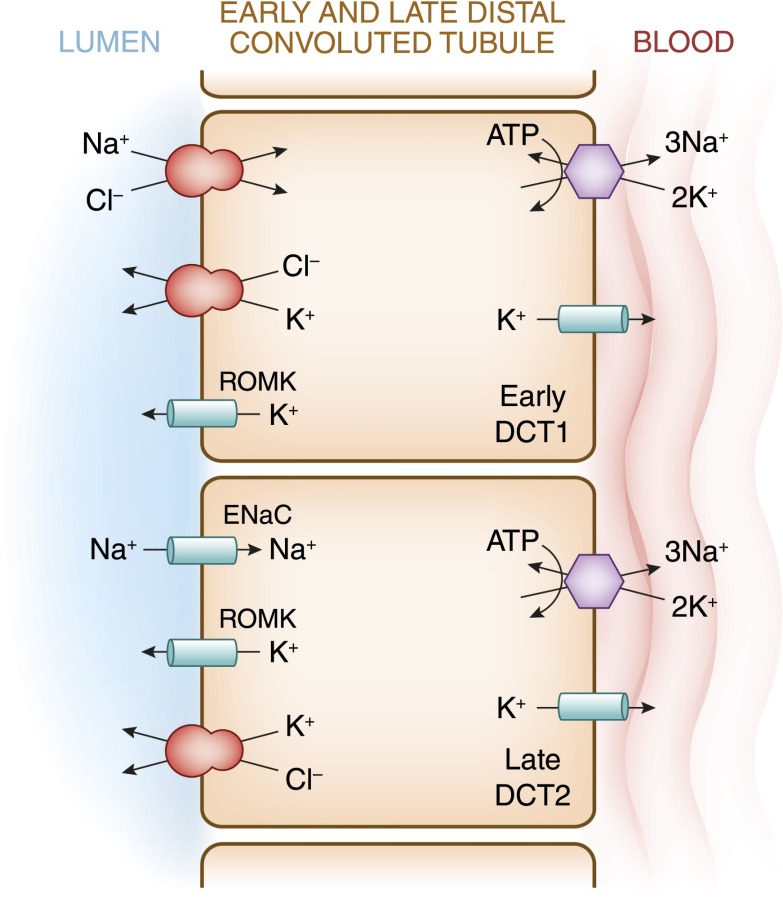
Figure 5.
A cell model for K+ transport in the distal convoluted tubule (DCT). In the early DCT, luminal Na+ uptake is mediated by the apically located thiazide-sensitive Na+-Cl− cotransporter. The transporter is energized by the basolateral Na+-K+-ATPase, which maintains intracellular Na+ concentration low, thus providing a favorable gradient for Na+ entry into the cell through secondary active transport. The cotransporter is abundantly expressed in the DCT1 but progressively declines along the DCT2. ROMK is expressed throughout the DCT and into the cortical collecting duct. Expression of the epithelial Na+ channel (ENaC), which mediates amiloride-sensitive Na+ absorption, begins in the DCT2 and is robustly expressed throughout the downstream connecting tubule and cortical collecting duct. The DCT2 is the beginning of the aldosterone-sensitive distal nephron (ASDN) as identified by the presence of both the mineralocorticoid receptor and the enzyme 11β-hydroxysteroid dehydrogenase II. This enzyme maintains the mineralocorticoid receptor free to only bind aldosterone by metabolizing cortisol to cortisone, the latter of which has no affinity for the receptor. Electrogenic-mediated K+ transport begins in the DCT2 with the combined presence of ROMK, ENaC, and aldosterone sensitivity. Electroneutral K+-Cl− cotransport is present in the DCT and collecting duct. Conditions that cause a low luminal Cl− concentration increase K+ secretion through this mechanism, which occurs with delivery of poorly reabsorbable anions, such as sulfate, phosphate, or bicarbonate.