ABSTRACT
The SpxA1 and SpxA2 (formerly SpxA and SpxB) transcriptional regulators of Streptococcus mutans are members of a highly conserved family of proteins found in Firmicutes, and they were previously shown to activate oxidative stress responses. In this study, we showed that SpxA1 exerts substantial positive regulatory influence over oxidative stress genes following exposure to H2O2, while SpxA2 appears to have a secondary regulatory role. In vitro transcription (IVT) assays using purified SpxA1 and/or SpxA2 showed that SpxA1 and, less often, SpxA2 directly activate transcription of some of the major oxidative stress genes. Addition of equimolar concentrations of SpxA1 and SpxA2 to the IVT reactions neither enhanced transcription of the tested genes nor disrupted the dominant role of SpxA1. Substitution of a conserved glycine residue (G52) present in both Spx proteins by arginine (SpxG52R) resulted in strains that phenocopied the Δspx strains. Moreover, addition of purified SpxA1G52R completely failed to activate transcription of ahpC, sodA, and tpx, further confirming that the G52 residue is critical for Spx functionality.
IMPORTANCE Streptococcus mutans is a pathogen associated with the formation of dental caries in humans. Within the oral cavity, S. mutans routinely encounters oxidative stress. Our previous data revealed that two regulatory proteins, SpxA1 and SpxA2 (formerly SpxA and SpxB), bear high homology to the Spx regulator that has been characterized as a critical activator of oxidative stress genes in Bacillus subtilis. In this report, we prove that Spx proteins of S. mutans directly activate transcription of genes involved in the oxidative stress response, though SpxA1 appears to have a more dominant role than SpxA2. Therefore, the Spx regulators play a critical role in the ability of S. mutans to thrive within the oral cavity.
INTRODUCTION
The oral cavity is colonized by hundreds of bacterial species, some of which contribute to overall oral health and others that are associated with disease, such as dental caries and periodontitis. Among the pathogenic oral bacteria, clinical evidence paired with in vitro and in vivo studies strongly associates Streptococcus mutans with dental caries onset and development (1, 2). For all organisms that inhabit the oral cavity, oxidative stresses are relevant threats for which defense mechanisms must be in place. Aside from the presence of hydrogen peroxide (H2O2) in oral care products, members of the mitis group of streptococci, which cohabit the dental plaque along with S. mutans, are net producers of H2O2 (3–5). Notably, there is an inverse correlation between the proportion of S. mutans and of members of the mitis group in health and disease, with high numbers of S. mutans organisms associated with disease and a high proportion of mitis streptococci associated with oral health (6, 7). Ultimately, the breakdown of H2O2 into other variants of reactive oxygen species (ROS) can disturb the integrity of DNA and proteins and thereby pose a significant threat to the viability of S. mutans.
Spx is a global regulator ubiquitously found in low-GC-content Gram-positive bacteria (Firmicutes), and it has been shown to function as a transcriptional activator of genes critical for oxidative stress survival (8). While the majority of work that has contributed to the understanding of Spx function has come from the model organism Bacillus subtilis, evidence is now accumulating that Spx proteins exert similar regulatory functions in bacterial pathogens, including S. mutans (9–15). Spx proteins do not have a DNA-binding domain but rather exert influence over transcription, positive or negative, by interacting with the C-terminal domain of the alpha subunit of the RNA polymerase (RNAP α-CTD) (16, 17). The method of regulation of these so-called appropriator proteins is different from the more common mechanism of transcriptional regulators that physically interact with the regulatory region of a target gene via a DNA-binding domain (18–20).
In previous work, we identified and characterized two Spx proteins in S. mutans, earlier referred to as SpxA and SpxB (11, 21). We demonstrated that both proteins bear strong homology to the well-studied B. subtilis Spx (8, 17). To eliminate any confusion with gene nomenclature due to the existence of the SpxB pyruvate oxidase in certain streptococci, we now adopt the Streptococcus pneumoniae nomenclature (13) and refer to SpxA and SpxB as SpxA1 and SpxA2, respectively. Both SpxA1 and SpxA2 possess a CXXC redox disulfide motif typical of Spx family regulators described to date (8–10, 13, 16, 22). This motif is thought to facilitate disulfide bond formation when exposed to an oxidizing environment, triggering a conformational change that ultimately facilitates interaction of Spx with the RNAP. In addition, SpxA1 and SpxA2 possess a conserved glycine residue (G52), which may be one of several residues involved in Spx–RNAP α-CTD interaction.
We previously constructed mutant strains bearing deletions of the spx genes in S. mutans, each singly (ΔspxA1 and ΔspxA2) or in combination (ΔspxA1 ΔspxA2). We observed that the ΔspxA1 strain exhibited strong stress-sensitive phenotypes, in particular toward oxidative stresses, which were often exacerbated in the double-mutant ΔspxA1 ΔspxA2 strain (11). Microarray analysis suggested that many genes involved in oxygen reduction and ROS detoxification were under positive regulation by SpxA1 and, to a minor extent, SpxA2 (11). Despite the association of Spx in oxidative stress responses in different bacteria, evidence that the regulatory effect exerted by Spx is direct has been limited to B. subtilis (23–25). Moreover, our previous studies suggest that the S. mutans SpxA1 is a more potent regulator than its SpxA2 paralogue (11, 26), although this observation had not been experimentally proven. In this study, we used quantitative reverse transcriptase real-time PCR (qRT-PCR) and in vitro transcription (IVT) assays to better understand the role of SpxA1 and SpxA2 in oxidative stress gene regulation. Our results provided unequivocal evidence that SpxA1 is required for optimal expression of some of the major oxidative stress genes through direct interaction with the RNAP. We also demonstrated that SpxA2 could contribute to transcription of oxidative stress genes, though its regulatory influence appears secondary to that of SpxA1. Finally, single amino acid substitutions confirmed the essentiality of the G52 residue for functionality of both SpxA1 and SpxA2 in S. mutans.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. S. mutans UA159 and its Δspx derivatives were routinely grown in brain heart infusion (BHI) at 37°C in a 5% CO2 atmosphere. When appropriate, erythromycin (Erm; 10 μg ml−1) or spectinomycin (1,000 μg ml−1) was added to the growth medium. For gene expression analysis, cultures were grown to an optical density at 600 nm (OD600) of 0.4, at which point control samples were harvested by centrifugation, while experimental samples were exposed to 0.5 mM H2O2 for 5 min before harvest. Harvested pellets were stored at −80°C until use.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotypea or function |
Source or reference |
|---|---|---|
| Streptococcus mutans strains | ||
| UA159 | Wild type | Laboratory stock |
| JL12 ΔspxA1 | spxA1::Spr | 21 |
| JL13 ΔspxA2 | spxA2::Ermr | 11 |
| JL21 ΔspxA1 ΔspxA2 | spxA1::Spr spxA2::Ermr | 11 |
| JL32 spxA1G52R | SpxA1G52R | This study |
| JL33 spxA2G52R | SpxA2G52R | This study |
| JL34 ΔspxA1 spxA2G52R | spxA::Spr SpxA2G52R | This study |
| Other bacterial strains | ||
| B. subtilis MH5636 | His-tagged RNAP | 42 |
| E. coli BL21(DE3) | Expression host strain | New England BioLabs |
| E. coli DH5α | Expression host strain | Invitrogen |
| S. gordonii DL-1 | Wild type | Laboratory stock |
| Plasmids | ||
| pET16B-SpxA1 | His-tagged SpxA1 | This study |
| pET16B-SpxA2 | His-tagged SpxA2 | This study |
| pSU20erm-lacG | Competency control | 27 |
| pMSP3535 | Expression in S. mutans | 31 |
| pMSP3535-SpxA1-His | Expression of SpxA1-His | This study |
| pMSP3535-SpxA2-His | Expression in SpxA2-His | This study |
| pMALc2x-SpxA1 | MBP-tagged SpxA1 | This study |
| pMALc2x-SpxA1G52R | MBP-tagged SpxA1G52R | This study |
Spr, spectinomycin resistance; Ermr, erythromycin resistance.
Creation of spxG52R strains.
A PCR-based site-directed mutagenesis strategy was used to create markerless point mutations in the S. mutans spxA1 and spxA2 genes. Specifically, primers (Table 2) were designed to introduce changes in the DNA sequence so that the GGG (spxA1) and GGA (spxA2) bases encoding glycine residues at position 52 of each protein were replaced by CGC and CGT, respectively, which both encode arginine. S. mutans UA159 was transformed with each PCR product in combination with the pSU20erm-lacG suicide plasmid encoding resistance to erythromycin (27). The plasmid was included for the purpose of identifying competent cells. Erm-resistant colonies were screened for a phenotype (changes in chain length) typical of ΔspxA1 or ΔspxA2 and then submitted for sequencing analysis of the appropriate gene to confirm the desired point mutation.
TABLE 2.
Primers used in this study
| Name | Sequence (5′–3′) | Application |
|---|---|---|
| 5′SpxA1pET-16B | AGGGGTAGTCATATGATGGTTACC | His-SpxA1 express |
| 3′SpxA1pET16B | GGGTAAGCGCTCGAGTTAGTCATCTTC | His-SpxA1 express |
| 5′SpxA2pET16B | GAAAGAGAGATCATATGATGATTAAAATTTA | His-SpxA2 express |
| 3′SpxA2pET16B | TCATTACTTCGACTCGAGTTATAAAGCTGC | His-SpxA2 express |
| 5′pET16BBamHI | TTGTTTAACTGGATCCGGAGATATACCATG | Release His-tag Spx |
| 3′pET16BXhoI | CGGGCTTTGTCTGCAAC | Release His-tag Spx |
| 5′spxA1pMAL | AAGGGGTAGTGAATTCATGGTTACC | MBP-SpxA1 express |
| 3′spxA1pMAL | GGGTAAGCGGGATCCTTAGTCATCTTC | MBP-SpxA1 express |
| 5′A1G52R-PCR1 | CTTTCCAGAGTCTCCTCAATTAGC | spxA1 G52R mutation |
| 3′A1G52R-PCR1 | GTCGAAATAATATCTTCTGTGCGGTTTTCTGTATAGG | spxA1 G52R mutation |
| 5′A1G52R-PCR2 | CCTATACAGAAACCGCACAGAAGATATTATTTCGAC | spxA1 G52R mutation |
| 3′A1G52R-PCR2 | GGTTTGACGACCGAAATCGGC | spxA1 G52R mutation |
| 5′A2G52R-PCR1 | GCAGGGAAATTGATTGATTCTGGCGC | spxA2 G52R mutation |
| 5′A2G52R-PCR1 | GCAGGGAAATTGATTGATTCTGGCGC | spxA2 G52R mutation |
| 3′A2G52R-PCR1 | GGATACAATGCTTTCAATACGATTTTCTGTTTTTG | spxA2 G52R mutation |
| 5′A2G52R-PCR2 | CAAAAACAGAAAATCGTATTGAAAGCATTGTATCC | spxA2 G52R mutation |
| 3′A2G52R-PCR2 | CAACATCATACTATAGTTATAGTACAAGC | spxA2 G52R mutation |
| sodART Fwd | GATGCTGAAACGATGACCCTTC | qRT-PCR, sodA |
| sodART Rev | CACATCAGCCAAAAGCACTTCC | qRT-PCR, sodA |
| tpxRT Fwd | CTCCATCTGCTTGGACGTGCTG | qRT-PCR, tpx |
| tpxRT Rev | GCAAGGGCAGCGTCATAGTTG | qRT-PCR, tpx |
| ahpCRT Fwd | ATGGTTTAGCACAACGTCGAAC | qRT-PCR, ahpC |
| ahpCRT Rev | TTGGCAGGGCAAACTTCTCC | qRT-PCR, ahpC |
| gorRT Fwd | ACCTGTAATGTTGGCTGTG | qRT-PCR, gor |
| gorRT Rev | CCTGACGATTTTGCTTCAAGAC | qRT-PCR, gor |
| noxRT Fwd | GGGTTGTGGAATGGCACTTTGG | qRT-PCR, nox |
| noxRT Rev | CAATGGCTGTCACTGGCGATTC | qRT-PCR, nox |
| dprRT Fwd | GAAGAAACAGTTGGCACATGGG | qRT-PCR, dpr |
| dprRT Rev | TTCCGTTTGAGCTGCTGTAAAG | qRT-PCR, dpr |
| trxART Fwd | TTGAAGCTGAAACGGCTAAGGG | qRT-PCR, trxA |
| trxART Rev | GCCTGCATAAGACATGGACCAC | qRT-PCR, trxA |
| trxBRT Fwd | AGTTGTTGGTGGTGGCGATTC | qRT-PCR, trxB1 |
| trxBRT Rev | CATTGGCAAAGGCACGTTCTTG | qRT-PCR, trxB1 |
| trxB2RT Fwd | GGAGAGGCACCAACAGCAG | qRT-PCR, trxB2 |
| trxB2RT Rev | ATGATGTGGAATGAACGACACG | qRT-PCR, trxB2 |
| 5′IVTsodA | GCTATTAAGAGCGGGACTTAC | IVT, sodA |
| 3′sodA | GCATGATGCTTATCATGATG | IVT, sodA |
| 5′tpx | GAGTGGTCAAAAAATAAACATATTTTTATTG | IVT, tpx |
| 3′tpx | CTGTCAGAGAAAAATCAGGTGCTGTATC | IVT, tpx |
| 5′ahpC | CTCTTTTGTTTTATGCTTCAAATTTTATTTTTAG | IVT, ahpC |
| 3′ahpC | GTTAACCGTAACAAATTCTCCTTGATG | IVT, ahpC |
| 5′mleS | GAATACAAGTTTAAAAGCAAATAGTTAAC | IVT, mleS |
| 3′mleS | GGCAAAAGACCAATAAGTCC | IVT, mleS |
Growth inhibition assay.
The ability of Streptococcus gordonii to inhibit growth of S. mutans via H2O2 production was assessed as described previously by Kreth et al. (28). Briefly, 8 μl of an overnight culture of S. gordonii DL-1 was spotted on the center of a BHI agar plate and incubated at 37°C for 16 h. The following day, 8-μl quantities of S. mutans cultures were spotted near the S. gordonii spot and incubated for an additional 16 h before visualizing the ability of the different S. mutans strains to grow in proximity of S. gordonii. To ascertain that any growth inhibition was due to the production of H2O2 by S. gordonii, a control condition was included in which 8 μl of catalase (0.75 μg μl−1) was immediately spotted on top of the S. gordonii culture.
Disc sensitivity assays.
Bacterial cultures were grown in BHI to an OD600 of 0.2, and aliquots (25 μl) of each culture were spread evenly over a quadrant of a 30-ml BHI plate with a sterile swab. Next, 6-mm filter paper discs (Whatman) were soaked with 20 μl of a 0.25% H2O2 or 0.2 M diamide solution and aseptically placed on top of the swabbed plate. Diameters of zones of growth inhibition were measured after 24 h of incubation at 37°C in a 5% CO2 atmosphere. Student's t test was performed to verify significant differences in sensitivity.
RNA analysis.
Total RNA was isolated as described previously (1). Briefly, RNA was isolated from homogenized S. mutans cells by repeated hot acid-phenol-chloroform extractions, and the nucleic acid was precipitated with 1 volume of ice-cold isopropanol and 1/10 volume of 3 M sodium acetate (pH 5) at 4°C overnight. RNA pellets were resuspended in nuclease-free H2O and treated with DNase I (Ambion) at 37°C for 30 min. The RNA was purified again using the RNeasy minikit (Qiagen), including a second on-column DNase treatment that was performed as recommended by the supplier. RNA concentrations were determined in triplicate using a Nanovue spectrophotometer (GE Healthcare) and run on an agarose gel to verify RNA integrity. For mRNA analysis, gene specific primers (Table 2) were designed using Beacon Designer 2.0 software (Premier Biosoft International). Reverse transcription and qRT-PCR were carried out according to protocols described elsewhere (1, 29). Student's t test was performed to verify significance of the qRT-PCR results.
Spx purification.
SpxA1 and SpxA2 were produced as recombinant His-tagged fusion proteins using the pET-16B expression vector (EMD Millipore). Both spxA1 and spxA2 were cloned using NdeI and XhoI restriction sites that had been engineered into primers flanking the coding regions of each gene (Table 2). Protein expression was induced by growing Escherichia coli DH5α in Luria-Bertani (LB) broth supplemented with 100 μg ml−1 of ampicillin at 37°C with agitation (200 rpm). After reaching OD600 of 0.5, cultures were transferred to 15°C and 0.4 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to induce production of the tagged protein overnight. SpxA1 protein was purified from the soluble fraction of cell lysate. Cell pellets were collected by centrifugation, resuspended in lysis buffer S (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole [pH 8.0]), and subjected to three 30-s cycles of homogenization using a bead beater (BioSpec Products), with chilling on ice between cycles. After centrifugation, purification of the recombinant proteins in the soluble supernatant was performed by column chromatography using nickel-nitrilotriacetic acid (Ni-NTA) resin (Qiagen). Recombinant SpxA1 was released from the resin with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole [pH 8.0]) and dialyzed in phosphate-buffered saline (PBS) containing 10% glycerol. For SpxA2, reasonable yields were obtained only when the protein was purified from the insoluble fraction and refolded. Cell pellets were resuspended in lysis buffer I (50 mM Tris-HCl, 0.1 mM EDTA, 0.1 mM dithiothreitol [DTT], 100 mM NaCl, 5% glycerol [pH 8.0]) and subjected to homogenization as described above. After centrifugation, the pellet was retained and resuspended in lysis buffer B containing 1% Triton X-100. The resuspended protein was denatured by the addition of 8 M urea and subjected to column chromatography using Ni-NTA resin, with 8 M urea included in the wash buffers. The purified SpxA2-His protein was refolded by stepwise dialysis with decreasing concentrations of urea, with a final dialysis step in the absence of urea. Protein concentrations were determined using the bicinchoninic acid assay (Sigma) (30).
To facilitate in vivo complementation and verify that the His-tagged Spx proteins retained activity, the coding region for the protein and N-terminal His tag were amplified from their respective constructs (pET16B-SpxA1 or pET16B-SpxA2). The amplicons were cloned into pMSP3535 (31) using BamHI and XhoI restriction sites that were engineered into the amplifying primers. The resulting plasmids, pMSP3535-SpxA1-His and pMSP3535-SpxA2-His, were confirmed by sequencing and then transformed into ΔspxA1 and ΔspxA2 S. mutans, respectively. Empty pMSP3535 was transformed into S. mutans UA159 as a control. Protein expression in pMSP3535 is driven by the nisA promoter upon addition of nisin to the culture. However, it has been shown that low-level protein expression may occur in the absence of nisin (31).
SpxA1 and SpxA1G52R were also produced as recombinant proteins fused to a maltose-binding protein (MBP) tag using the pMALc2X expression vector (New England BioLabs). The spxA1 and mutated spxA1G52R genes were cloned into pMALc2X using BamHI and EcoRI restriction sites that had been engineered into primers flanking the coding regions of each gene (Table 2). Protein expression was induced by growing E. coli strain DH5α in LB broth supplemented with 1% (wt/vol) glucose and 100 μg ml−1 of ampicillin at 37°C with agitation (200 rpm). The resulting cultures were grown until an OD600 of 0.5 was reached, at which point the cultures were transferred to 15°C, and 0.3 mM IPTG was added to induce production of the tagged protein overnight. Cell pellets were collected by centrifugation, resuspended in column buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA), and homogenized in a bead beater as described above. Purification of the recombinant proteins was performed by column chromatography using amylose resin (New England BioLabs). Recombinant SpxA1-MBP and SpxA1G52R-MBP were eluted in column buffer containing 10 mM maltose and dialyzed in PBS containing 10% glycerol.
RNAP purification.
A crude extract of S. mutans RNAP was obtained based upon the methods described by Seepersaud et al. for isolation of Streptococcus agalactiae RNAP, which utilizes the binding properties of heparin to enrich for RNAP (32). Briefly, S. mutans cells were grown in BHI to an OD600 of 0.5, harvested by centrifugation, resuspended in protoplast preparation buffer (0.3 M potassium phosphate buffer, pH 7.0; 40% sucrose; 0.5 U μl−1 of mutanolysin), and incubated at 37°C for 90 min. Protoplasts were harvested, and the pellets were resuspended in lysis buffer (50 mM Tris HCl, 10 mM MgCl2, 0.1 mM DTT, 0.1 mM EDTA, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride [PMSF] [pH 8.0]) and then subjected to three 15-s rounds of sonication, with chilling on ice between cycles. Following centrifugation, supernatants were applied to an Affi-Gel heparin resin (Bio-Rad) and eluted with a gradient of 0.1 to 1 M NaCl. The elutions were then run on 10% SDS-PAGE, the fractions containing a visible β subunit (∼134 kDa) were pooled, and the salt concentration was adjusted to 0.1 M NaCl by buffer exchange. The protein was then applied to Macro-Prep High-Q ion-exchange resin (Bio-Rad) and eluted with a gradient of 0.1 to 0.8 M NaCl. The desired fractions were again pooled, dialyzed in 10 mM Tris HCl, 10 mM MgCl2, 100 mM NaCl, 0.1 mM EDTA, and 50% glycerol (pH 8.0), and stored at −20°C.
His-tagged, σA-depleted RNAP was purified from B. subtilis strain MH5636 as described by Lin and Zuber (23). Briefly, cultures were grown in 2× yeast extract-tryptone (2× YT) medium supplemented with 5 μg ml−1 of chloramphenicol at 37°C under agitation to an OD600 of 0.9, harvested, and kept at −80°C until use. Protein purification was performed in multiple steps using Ni-NTA resin, an Affi-Gel heparin column, and a High-Q ion-exchange column (23). The purified RNAP was stored at −20°C in buffer containing 10 mM Tris-HCl (pH 7.8), 100 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, and 50% glycerol.
In vitro transcription assays.
A linear DNA template for each gene of interest was generated by PCR using primers designed to amplify the promoter region as well as about 70 to 100 bp of the coding region (Table 2). Following amplification, the DNA fragments were purified with a QIAquick PCR purification kit (Qiagen). The In vitro transcription (IVT) reactions were performed as described by Lin and Zuber (23). Briefly, a 10 nM concentration of each individual purified promoter template, 95 nM S. mutans crude RNAP or 25 nM B. subtilis RNAP, and 25 nM B. subtilis σA (a gift from P. Zuber, Oregon Health Science Center) were incubated for 10 min at 37°C with or without purified His- or MBP-tagged SpxA1, His-tagged SpxA2, His-tagged SpxA1G52R, or His-tagged S. mutans CodY (a gift from R. Quivey, University of Rochester) in 10 mM Tris-HCl (pH 8.0), 50 mM NaCl, 5 mM MgCl2, and bovine serum albumin (BSA; 50 μg ml−1) supplemented with 2 U of RNase inhibitor (Applied Biosystems) to a final volume of 20 μl. The Spx or CodY proteins were provided at 75 nM unless otherwise specified. A nucleotide mixture (200 mM ATP, GTP, and CTP, 10 mM UTP, and 5 μCi of [α-32P]UTP) was added, and the incubation proceeded for an additional 3 to 12 min. Stop solution (1 M ammonium acetate, 0.1 mg ml−1 of yeast RNA, 0.03 M EDTA) was added and the mixture was precipitated with ethanol at 4°C overnight. The nucleotide pellet was resuspended in formamide dye (0.3% xylene cyanol, 0.3% bromophenol blue, and 12 mM EDTA dissolved in formamide). The samples were heated at 90°C for 2 min and then placed on ice before application to an 8% polyacrylamide–8 M urea gel. The transcripts were visualized with Bio-Rad Quantity One Fx software following overnight exposure to an Imaging Screen-K (Bio-Rad). Densitometry was performed using ImageJ software (http://imagej.nih.gov/ij/).
RESULTS
Deletion of SpxA1 and, to a lesser extent, SpxA2 impairs the ability of S. mutans to respond to oxidative stress.
Previously, we demonstrated that several genes critical for responding to oxidative stresses were expressed at significantly reduced levels in the ΔspxA1 and ΔspxA1 ΔspxA2 strains under nonstressful conditions compared to the parent UA159 strain (11). While these genes were mostly absent in the microarray analysis, qRT-PCR validation revealed that many of these genes are also repressed in the ΔspxA2 mutant, though typically not to the same extent as in the ΔspxA1 mutants. These initial data suggested that SpxA1 is the major regulator of oxidative stress genes in S. mutans, while SpxA2 appears to perform a backup regulatory role. Alternatively, SpxA2 may exert a more prominent control over oxidative stress gene expression under conditions that are yet to be determined. In this study, we asked if these differences in transcription were restricted to basal expression levels or whether the differences would become more noticeable following exposure to an oxidative stressor. To assess this possibility, we exposed mid-logarithmic cultures of the UA159, ΔspxA1, ΔspxA2, and ΔspxA1 ΔspxA2 strains to 0.5 mM H2O2 for 5 min and compared the transcription patterns of key oxidative stress genes with untreated (control) cultures (Fig. 1). Transcription expression levels of nine well-established oxidative stress genes were studied for all four strains (sodA, superoxide dismutase [SMU.629]; tpx, thiol peroxidase [SMU.924]; ahpC, alkyl hydroperoxide reductase [SMU.764]; gor, glutathione reductase [SMU.838]; nox, NADH oxidase [SMU.1117]; dpr, peroxide resistance protein [SMU.540]; trxA, thioredoxin [SMU.1869]; trxB1, thioredoxin reductase [SMU.463]; and trxB2, thioredoxin reductase [SMU.869]). As expected, the parent strain showed increased transcription of almost all of the genes following exposure to H2O2; the trxA and trxB2 genes were the only exceptions to this trend. Additionally, among the untreated samples, UA159 showed the greatest expression level for all of these genes, with the exception of trxB2, which was not regulated by Spx. This trend among the untreated samples reveals that SpxA1 and SpxA2 are important for maintaining basal expression levels of the major oxidative stress genes even in the absence of stress. For simplicity, genes that showed similar patterns of expression have been grouped together and are described below.
FIG 1.
Mutation of spxA1 abolishes the ability of S. mutans to transcriptionally respond to the presence of H2O2. S. mutans UA159, ΔspxA1, ΔspxA2, and ΔspxA1 ΔspxA2 strains were grown in BHI to an OD600 of 0.4. At that point, control cultures (−) were harvested, while the remaining samples (+) were exposed to 0.5 mM H2O2 for 5 min before harvest. RNA was extracted from the cell pellets and used in qRT-PCR analysis to detect transcription of selected oxidative stress genes. Bars represent the relative copy number detected for each gene. Student's t test was performed to verify significance of the data. #, significant difference from the same strain when untreated; Δ, significant difference from untreated UA159; *, significant difference from UA159 after H2O2 treatment. Data shown represent averages and SDs from three replicate samples (#, Δ, and *, P ≤ 0.05; ##, ΔΔ, and **, P ≤ 0.005; ###, ΔΔΔ, and ***, P ≤ 0.0005).
Group I (sodA, tpx, ahpC, and gor).
Among the group I genes, basal levels of expression in both the ΔspxA1 and ΔspxA1 ΔspxA2 strains were noticeably reduced compared to those in the parent and, for sodA and tpx, the ΔspxA2 strain. The most extreme example of this trend was seen for ahpC, in which basal level expression was decreased 40-fold (ΔspxA1) or 65-fold (ΔspxA1 ΔspxA2) compared to that in the parent strain. For all genes in this grouping, exposure to H2O2 always resulted in increased expression in both UA159 and ΔspxA2. The preeminent role of SpxA1 in transcriptional activation of these genes becomes even more striking due to the complete lack of transcriptional activation in the ΔspxA1 and ΔspxA1 ΔspxA2 strains after H2O2 exposure.
Group II (nox and dpr).
One trend that separates nox and dpr from the previous grouping of genes is that the levels of expression among the untreated Δspx strains, including the ΔspxA2 strain, were not dramatically different. These mutants showed lower basal levels of expression than did UA159, a minimum of a 2.8-fold difference. Similar to the trends seen in the previous grouping of genes, exposure to H2O2 increased expression of both nox and dpr in the UA159 and ΔspxA2 strains. Although expression of nox and dpr was induced in the ΔspxA2 strain following exposure to the stress, the mRNA copy numbers were significantly reduced compared to those with the parent strain. These differences may be attributed to the low basal expression for nox and dpr in the ΔspxA2 strain. As in the previous grouping of genes, the ΔspxA1 and ΔspxA1 ΔspxA2 strains were essentially unresponsive to H2O2.
Group III (trxA).
Expression levels of trxA were unique in that basal levels of expression were quite different among all four strains: expression was greatest in UA159, followed by the ΔspxA1 strain (8-fold decrease), the ΔspxA2 strain (74-fold decrease), and, finally, the ΔspxA1 ΔspxA2 strain (337-fold decrease). The trxA gene is the only gene included in this study for which the basal level of expression was notably greater in the ΔspxA1 strain than in the ΔspxA2 strain. Unexpectedly, transcription of trxA did not increase in the parent UA159 strain upon exposure to H2O2, but rather it showed a slight decrease (2.6-fold). While the ΔspxA1 and ΔspxA1 ΔspxA2 strains were essentially unresponsive to H2O2, expression of trxA increased 8.4-fold in the ΔspxA2 strain.
Group IV (trxB1 and trxB2).
The group IV genes from the thioredoxin system each showed unique patterns in expression and were not easily grouped with the other genes included in this study. Although basal levels of trxB1 were not statistically different between strains, H2O2 induction was, as in the other cases, SpxA1 dependent. Finally, the second putative thioredoxin reductase trxB2 does not seem to be under the regulation of any of the two Spx proteins, and its expression was not altered during exposure to H2O2.
Collectively, these results demonstrate that both SpxA1 and SpxA2 participate in the regulation of oxidative stress genes. Largely, the data confirm our previous microarray analysis (11) and point to SpxA1 as the major transcriptional regulator of oxidative stress genes, while SpxA2 may play a more relevant role in maintaining basal levels of expression.
SpxA1 directly regulates transcription of oxidative stress genes.
The qRT-PCR analysis supported a role for SpxA1 as a major regulator of oxidative stress genes. However, it did not rule out a role for SpxA2, which appears to be important for maintaining basal levels of transcription. With the exception of the nox gene, which we have recently showed is directly activated by SpxA1 (26), it is not known whether the regulatory effects exerted by the S. mutans Spx proteins over other genes are direct or indirect. To answer this question, we performed IVT reactions using either a crude extract of the S. mutans RNAP or purified B. subtilis RNAP (results were not influenced by RNAP source), and the promoter regions of three major oxidative stress genes (ahpC, sodA, and tpx), in the presence or absence of purified His-tagged SpxA1 or SpxA2.
To confirm that the addition of the His tag did not have a negative impact upon function of Spx, we expressed the His-tagged SpxA1 and SpxA2 proteins in S. mutans using the pMSP3535 plasmid. Expression of His-tagged SpxA1 in the ΔspxA1 strain resulted in alleviation of the oxidative stress phenotype, as measured by sensitivity to filter paper discs impregnated with H2O2 (Fig. 2A). We previously reported distinctive chain length phenotypes in the Δspx deletion strains when observed under the microscope whereby the ΔspxA1 strain had a tendency to form longer chains than UA159, while the ΔspxA2 strain was impaired in chain formation (11). Expression of SpxA1-His or SpxA2-His in the respective deletion mutant strain reverted the chain length phenotype, confirming that both His-tagged Spx proteins retain their normal activities in vivo (Fig. 2B).
FIG 2.
His-tagged SpxA proteins are active in vivo. His-tagged SpxA1 or SpxA2 was cloned into pMSP3535, allowing complementation of ΔspxA1 or ΔspxA2 in S. mutans. (A) Early logarithmic-phase cultures of S. mutans UA159 plus pMSP3535, the ΔspxA1 mutant plus pMSP3535, or the ΔspxA1 mutant plus pMSP3535-SpxA1-His were swabbed onto a BHI agar plate, upon which filter paper discs impregnated with 0.25% H2O2 were placed. Diameters of zones of growth inhibition were measured after 24 h of incubation at 37°C and 5% CO2. Data shown represent averages and SDs from three replicate samples (*, P ≤ 0.05; **, P ≤ 0.005). (B) Chain lengths of mid-logarithmic phase cultures of S. mutans UA159, UA159 + pMSP3535, the ΔspxA1 mutant, the ΔspxA1 mutant plus pMSP3535-SpxA1-His, the ΔspxA2 mutant, and the ΔspxA2 mutant plus pMSP3535-SpxA2-His were observed by phase-contrast microscopy.
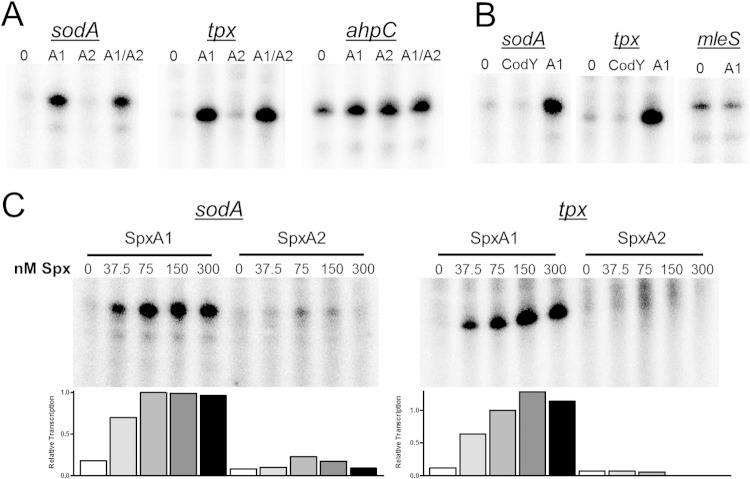
Results of the IVT reactions undoubtedly demonstrate that addition of SpxA1 dramatically enhanced transcription of all three genes tested (Fig. 3A). In fact, in the cases of sodA and tpx, the transcript was barely detectable unless SpxA1 was added to the reaction mixture. IVT reactions for the selected genes were also performed with SpxA2 supplied instead of SpxA1. Under the initial conditions tested, SpxA2 increased transcript abundance for ahpC but not sodA or tpx (Fig. 3A). To consider the possibility that SpxA1 and SpxA2 either compete or cooperate with each other, the proteins were also provided together in equimolar concentrations, each at half the concentration as when provided singly. Our results indicate that the two Spx proteins do not cooperate or compete with each other, at least under the conditions tested. To be certain that enhanced transcription by Spx was specific, control reactions using recombinant His-tagged S. mutans CodY were performed. CodY is a regulator involved in branched-chain amino acid biosynthesis and is not known to have a role in oxidative stress gene regulation. As expected, CodY did not enhance transcription of sodA or tpx (Fig. 3B). Finally, IVT reactions were also performed with the promoter region of the mleS gene, involved in malolactic fermentation and not expected to be under Spx regulation. Transcription of mleS was not enhanced by SpxA1 addition (Fig. 3B).
FIG 3.
SpxA1 and SpxA2 specifically enhance transcription of oxidative stress genes. In vitro transcription (IVT) reactions were performed by incubating selected DNA templates with S. mutans RNAP and nucleotides including [α-32P]UTP in the absence (0) or presence of purified His-tagged protein: SpxA1 (A1), SpxA2 (A2), and SpxA1 and SpxA2 combined (A1/A2). Reactions were performed at 37°C. Radiolabeled RNA transcripts were precipitated, applied to 8% urea PAGE, and visualized by exposure to a phosphorimager screen. (A) IVT reactions performed with template DNA for ahpC, sodA, or tpx. (B) His-tagged CodY failed to enhance transcription of sodA or tpx, and SpxA1 failed to enhance transcription of mleS. (C) IVT reactions were performed with increasing amounts of SpxA1 or SpxA2 using the sodA or tpx DNA templates. Representatives of three or more independent reactions are shown. Densitometry was performed with ImageJ software.
To rule out the possibility that simply a greater amount of SpxA2 is required to induce transcription from the tested genes, additional IVT reactions with sodA and tpx were performed using increasing concentrations of Spx. By adding increasing amounts of SpxA2, a very small increase in sodA transcription but not tpx could be observed in these reactions (Fig. 3C). Despite the high level of homology between SpxA1 and SpxA2, it is possible that SpxA2 requires a yet-to-be-determined environmental cofactor or partner protein to exert strong regulatory effects on gene transcription. Alternatively, the control and scope of SpxA2 regulation of oxidative stress genes may be much more limited those with SpxA1. Our previous phenotypic observations using the ΔspxA1 and ΔspxA2 strains (11) support the latter.
Substitution of a single residue of the Spx proteins results in strains that behave similarly to the Δspx strains.
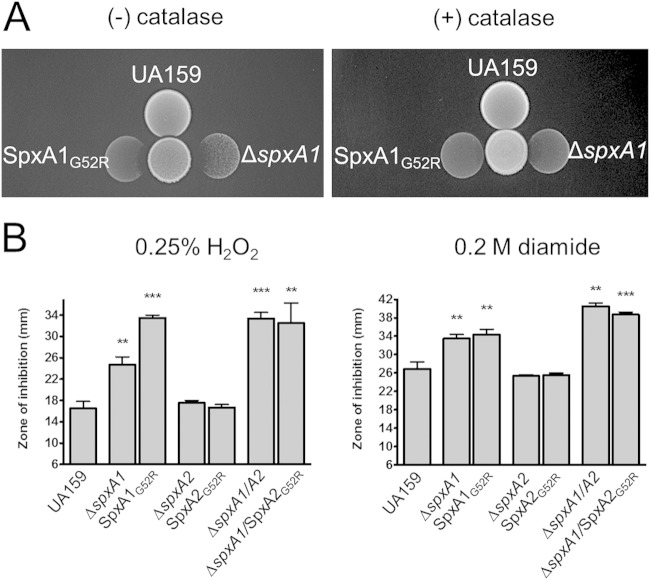
The Spx proteins in Firmicutes are highly similar, and a conserved glycine residue at position 52 (G52) appears to be critical for interaction with the RNAP (16, 17). To investigate the importance of this residue in S. mutans, we created point mutations in SpxA1 and SpxA2, such that the G52 residue of each protein was replaced with an arginine (respectively, spxA1G52R and spxA2G52R strains). If the G52 residue is in fact essential, the spxA1G52R and spxA2 G52R strains should phenocopy the ΔspxA1 and ΔspxA2 strains. As a means of verifying disrupted Spx function in these new strains, we again took advantage of the chain length phenotypes that we have described for the Δspx strains (11). Microscopic observations indicated that the spxA1G52R and spxA2G52R strains displayed chain length phenotypes similar to those described for their respective deletion mutant strains (Fig. 4), suggesting that substitution of the G52 residue by an arginine renders the Spx proteins inactive. In fact, the spxA1G52R strain was as sensitive to diamide or to the peroxigenic oral commensal S. gordonii as the Δspx strain (Fig. 5).
FIG 4.

Chain length phenotype of spxG52R strains is identical to that of ΔspxA strains. Overnight cultures of S. mutans UA159, ΔspxA1, and ΔspxA2 strains and variants with substitutions of the G52 residue (spxA1G52R and spxA2G52R) were visualized by light microscopy.
FIG 5.
The spxG52R strains phenocopied the ΔspxA strain in oxidative stress assays. (A) Inhibition assay reveals that ΔspxA1 S. mutans is sensitive to the H2O2 produced by S. gordonii (center spot). A strain in which the G52 of SpxA1 has been replaced with arginine (spxA1G52R) is equally sensitive. The assay was repeated with catalase overlaid onto the S. gordonii spot to inactivate the H2O2, resulting in loss of sensitivity. (B) Early logarithmic-phase cultures of S. mutans UA159, ΔspxA1, spxA1G52R, ΔspxA2, spxA2G52R, ΔspxA1 ΔspxA2, and ΔspxA1 spxA2G52R strains were swabbed onto a BHI agar plate, upon which filter paper discs impregnated with 0.25% H2O2 or 0.2 M diamide were placed. Diameters of zones of growth inhibition were measured after 24 h of incubation at 37°C and 5% CO2. Data shown represent averages and SDs from three replicate samples. **, P ≤ 0.005; ***, P ≤ 0.0005.
Our earlier characterizations revealed that the ΔspxA2 strain does not display the hypersensitivity to oxidative stresses that was observed for the ΔspxA1 strain. However, we had also demonstrated that the ΔspxA1 ΔspxA2 double mutant was even more sensitive to oxidative stress than the ΔspxA1 single mutant (11). To further test if replacement of the G52 residue was just as disruptive to the normal functioning of SpxA2, we deleted the spxA1 gene in the spxA2G52R strain, thereby creating a double ΔspxA1 spxA2G52R strain. We then used disc diffusion assays to test the sensitivity of the strains to H2O2 or diamide. In both cases, as previously observed, the ΔspxA2 strain showed a similar level of resistance as UA159. The ΔspxA1 and ΔspxA1 ΔspxA2 strains, however, demonstrated statistically significant heightened sensitivity to both compounds. The spxA1G52R and ΔspxA1 spxA2G52R strains showed sensitivities nearly identical to those of the corresponding single and double deletion strains. In the ΔspxA1 background, replacement of the G52 residue in SpxA2 resulted in a strain that was indistinguishable from the double Δspx strains (Fig. 5). Therefore, our results convey the importance of a single residue by demonstrating that replacing the glycine residue at position 52 with arginine disrupts the functionality of both SpxA1 and SpxA2.
The G52 residue is essential for SpxA1-dependent regulation.
The physiological characterization of the strains bearing SpxG52R point mutations (Fig. 5) demonstrated the importance of the G52 residue for Spx functionality. Next, we tested whether the G52 residue was indeed critical for SpxA1 to exert its effect upon gene transcription. To this end, we performed the IVT reactions with MBP-tagged versions of SpxA1 or the SpxA1G52R variant. Despite the presence of the large MBP tag, addition of SpxA1 but not of SpxA1G52R enhanced transcription of ahpC, sodA, and tpx (Fig. 6). In fact, cleavage of the MBP tag did not augment transcription (data not shown). These results confirmed that, as observed in B. subtilis, an intact G52 residue is critical for SpxA1 to exert its transcriptional influence over oxidative stress genes.
FIG 6.
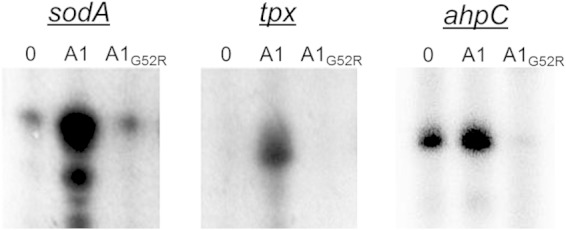
Enhanced gene transcription requires intact SpxA1 protein. In vitro transcription reactions were performed with the regulatory regions of ahpC, sodA, or tpx incubated with B. subtilis RNAP without SpxA1 (0), with MBP-tagged SpxA1 (A1), or with MBP-tagged SpxA1G52R (A1G52R). Radiolabeled RNA transcripts were precipitated, applied to 8% urea PAGE, and visualized by exposure to a phosphorimager screen. Representatives of three or more independent reactions are shown.
DISCUSSION
Mounting evidence indicates that transcriptional regulation by Spx is critical for the ability of low-GC Gram-positive bacteria to cope with oxidative stress (9, 11, 12, 15, 16, 22, 26, 33–36). However, proof of direct regulation by Spx in bacteria other than B. subtilis has been limited to the nox gene of S. mutans (26). Moreover, while some species, such as Enterococcus faecalis and Streptococcus aureus, appear to encode only one copy of a bona fide Spx regulator, streptococcal species and, more recently, Bacillus anthracis were shown to encode two Spx proteins, dubbed SpxA1 and SpxA2 (11, 15, 33). From these studies, transcriptomic and phenotypic characterization suggests that the two Spx paralogues have overlapping regulatory functions, although SpxA1 appears to exert a dominant role in oxidative stress gene regulation. Despite this progress, an understanding of how the two Spx proteins interact with the RNAP to activate transcription and even definitive proof that SpxA2 can bind to the RNAP are missing.
In this study, we exposed cells of the parent and Δspx strains to H2O2 stress, which allowed for a better appreciation of the role of each Spx protein in oxidative stress gene regulation. The results confirmed not only that the basal expression levels of the bulk of these genes (ahpC, dpr, gor, nox, sodA, tpx, trxA, and trxB1) are reduced when SpxA1 is absent but also that the strains lacking SpxA1 are completely unable to mount a transcriptional response toward oxidative stress. The main function of SpxA2 appears to be in the maintenance of basal levels of expression, rather than during acute oxidative stress conditions. These results fit well with our own previous data and recent studies with the bacterial pathogens Streptococcus suis and Bacillus anthracis, both possessing two Spx proteins (11, 15, 33). In the case of S. suis, an ΔspxA1 strain was considerably more sensitive to growth under oxidative conditions, while the ΔspxA2 strain was more sensitive to detergents or salt (15). Similarly, SpxA1 of B. anthracis was most critical for tolerance of H2O2, though, as in S. mutans, simultaneous disruption of both spxA1 and spxA2 resulted in extreme sensitivity to diamide (33).
By using IVT assays, we obtained direct evidence that both Spx proteins can serve as positive transcriptional regulators. In agreement with our previous microarray study (11) and transcriptional profiling of H2O2-treated cells, SpxA1 is the major regulator governing transcription of oxidative stress genes. Among the three genes included in the IVT analysis, ahpC, sodA, and tpx, the contribution of SpxA2 to transcription was evident only for ahpC, although increasing amounts of SpxA2 resulted in modest transcription of sodA. It is possible that SpxA2 requires a cofactor(s), an adaptor protein(s), or specific environmental conditions that were lacking in our in vitro assays. Another plausible scenario is that the affinity of the SpxA2-RNAP complex to the regulatory region of oxidative stress genes is weak compared to that of the SpxA1-RNAP complex. Along these lines, the primary targets of S. mutans SpxA2 transcriptional control may lie beyond the boundaries of the oxidative stress response. For example, our microarray data suggested a role for SpxA2 in cell wall homeostasis (11), and Spx regulation has been implicated in antibiotic resistance, biofilm formation, and salt and SDS stress (10, 12, 15, 35, 37, 38). It is conceivable that SpxA2 evolved to control genetic traits that are distinct from SpxA1, and yet it retained low affinity for the regulatory region of oxidative stress genes, thereby serving as a backup regulator when SpxA1 is not available. In the future, efforts to identify gene targets strongly dependent on SpxA2 for expression may shed new light on the cellular significance of SpxA2.
Another interesting possibility is that the two Spx proteins could work in concert in the activation of oxidative stress genes. However, recent work with B. subtilis revealed that Spx binds to the RNAP α-CTD as monomer (23), different than the initial belief that it bound as a dimer (24). If this is also true in S. mutans, then another possibility is that the two Spx proteins compete for binding to the RNAP. IVT assays in which SpxA1 and SpxA2 were provided in equal amounts indicated that neither cooperation nor competition between the two Spx proteins for RNAP binding seems to occur, although these observations are based on in vitro evidence and limited to only three genes.
In B. subtilis, replacement of the G52 residue by arginine (G52R) abolished the ability of Spx to exert transcriptional control, due to an inability to interact with the RNAP (24, 39). This interaction also requires conservation on the part of RNAP, and work in the Zuber lab demonstrated that certain amino acid substitutions in the alpha subunit of the RNAP also abolished this interaction (23, 40). The present study extends the importance of the G52 residue for Spx-stimulated transcription in S. mutans, as the spxA1G52R and spxA2G52R strains were phenotypically identical to their respective spxA1 and spxA2 deletion strains. As expected, no increase in transcription was observed in IVT reactions performed with the SpxA1G52R variant. In this case, it is possible that steric disruption of the G52R variant alters the conformation of the protein so that upon interaction with the RNAP, the SpxA1G52R variant causes a repositioning of the RNAP that does not favor transcription. Given that arginine is a considerably larger amino acid than glycine, it is possible that replacement of the G52 residue with a smaller amino acid such as alanine or valine would not result in the same outcome. Nevertheless, the substitution described in this study clearly demonstrates that interfering with the architecture of the S. mutans Spx proteins disrupts their normal function, likely due to inability to properly interact with the RNAP.
While the CXXC motif is central for redox control of Spx, recent work uncovered additional critical residues in the B. subtilis Spx. For example, mutational analysis suggests that the R92 residue is also important for redox control (24, 41). While the S. mutans SpxA1 possesses the R92 residue, the arginine is replaced by a serine (S92) in SpxA2. This is also the case in other streptococcal species such as S. gordonii, S. pneumoniae, and S. pyogenes, with one Spx protein (SpxA1) containing an R92 residue and the second (SpxA2) containing S92. We have hypothesized that Spx proteins harboring the R92 residue respond more strongly to environmental redox conditions and could be the basis for the differing roles that we have observed for the two Spx proteins of S. mutans. Studies to address the importance of the CXXC motif as well as the R92 residue to Spx functionality are under way.
A major challenge in the identification of Spx-regulated gene targets is the lack of a consensus DNA binding motif. In B. subtilis, an AGCA motif, located at the −44 region, was shown to be required for Spx-dependent transcription of trxA and trxB, with the less stringent a/tGCa/t motif (letters in lowercase denote less conserved residues) associated with a greater number of genes (20, 24, 41). In a separate study, standard computational searches for consensus Spx-RNAP binding motifs in B. subtilis were unsuccessful (36). The second study concluded that in lieu of a traditional consensus motif, promoters of genes regulated by Spx displayed extended −35 and −10 elements. Especially noticeable was an extension of the −35 element in genes that were positively, as opposed to negatively, regulated by Spx. That study also noted the AGCA element upstream of the −35 element, but only for about 10% of the Spx-regulated genes (36). We searched for the AGCA motif in S. mutans and found it in only three of the genes involved in the present study (nox, sodA, and trxA), suggesting that the cis-acting site directing SpxA gene regulation may vary from species to species, or even from one gene to another. Mutational analysis of the promoter region of targeted genes will likely prove useful in identifying sequences that are critical for Spx transcriptional activation of S. mutans.
ACKNOWLEDGMENTS
This study was supported by NIH-NIDCR award DE019783 (J.A.L.). I.R.-R. was also supported by the NIDCR-NIH Training Program in Oral Sciences (T32 DE07165).
B. subtilis MH5636 and purified σA were kindly provided by Peter Zuber, Oregon Health and Science Center. His-tagged S. mutans CodY was kindly provided by Robert Quivey, University of Rochester. We thank the anonymous reviewers for helpful suggestions.
REFERENCES
- 1.Lemos JA, Burne RA. 2008. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol Rev 50:353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Ge X, Dou Y, Wang X, Patel JR, Xu P. 2011. Identification of hydrogen peroxide production-related genes in Streptococcus sanguinis and their functional relationship with pyruvate oxidase. Microbiology 157:13–20. doi: 10.1099/mic.0.039669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakubovics NS, Gill SR, Vickerman MM, Kolenbrander PE. 2008. Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol Ecol 66:637–644. doi: 10.1111/j.1574-6941.2008.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu L, Kreth J. 2012. The role of hydrogen peroxide in environmental adaptation of oral microbial communities. Oxid Med Cell Longev 2012:717843. doi: 10.1155/2012/717843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kreth J, Merritt J, Shi W, Qi F. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J Bacteriol 187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuramitsu HK, He X, Lux R, Anderson MH, Shi W. 2007. Interspecies interactions within oral microbial communities. Microbiol Mol Biol Rev 71:653–670. doi: 10.1128/MMBR.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuber P. 2004. Spx-RNA polymerase interaction and global transcriptional control during oxidative stress. J Bacteriol 186:1911–1918. doi: 10.1128/JB.186.7.1911-1918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Ge X, Wang X, Patel JR, Xu P. 2012. SpxA1 involved in hydrogen peroxide production, stress tolerance and endocarditis virulence in Streptococcus sanguinis. PLoS One 7:e40034. doi: 10.1371/journal.pone.0040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajfasz JK, Mendoza JE, Gaca AO, Miller JH, Koselny KA, Giambiagi-Demarval M, Wellington M, Abranches J, Lemos JA. 2012. The Spx regulator modulates stress responses and virulence in Enterococcus faecalis. Infect Immun 80:2265–2275. doi: 10.1128/IAI.00026-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kajfasz JK, Rivera-Ramos I, Abranches J, Martinez AR, Rosalen PL, Derr AM, Quivey RG, Lemos JA. 2010. Two Spx proteins modulate stress tolerance, survival, and virulence in Streptococcus mutans. J Bacteriol 192:2546–2556. doi: 10.1128/JB.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pamp SJ, Frees D, Engelmann S, Hecker M, Ingmer H. 2006. Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. J Bacteriol 188:4861–4870. doi: 10.1128/JB.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turlan C, Prudhomme M, Fichant G, Martin B, Gutierrez C. 2009. SpxA1, a novel transcriptional regulator involved in X-state (competence) development in Streptococcus pneumoniae. Mol Microbiol 73:492–506. doi: 10.1111/j.1365-2958.2009.06789.x. [DOI] [PubMed] [Google Scholar]
- 14.Turner MS, Tan YP, Giffard PM. 2007. Inactivation of an iron transporter in Lactococcus lactis results in resistance to tellurite and oxidative stress. Appl Environ Microbiol 73:6144–6149. doi: 10.1128/AEM.00413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng C, Xu J, Li J, Hu L, Xia J, Fan J, Guo W, Chen H, Bei W. 2014. Two Spx regulators modulate stress tolerance and virulence in Streptococcus suis serotype 2. PLoS One 9:e108197. doi: 10.1371/journal.pone.0108197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano S, Erwin KN, Ralle M, Zuber P. 2005. Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol Microbiol 55:498–510. [DOI] [PubMed] [Google Scholar]
- 17.Newberry KJ, Nakano S, Zuber P, Brennan RG. 2005. Crystal structure of the Bacillus subtilis anti-alpha, global transcriptional regulator, Spx, in complex with the alpha C-terminal domain of RNA polymerase. Proc Natl Acad Sci U S A 102:15839–15844. doi: 10.1073/pnas.0506592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell EA, Westblade LF, Darst SA. 2008. Regulation of bacterial RNA polymerase sigma factor activity: a structural perspective. Curr Opin Microbiol 11:121–127. doi: 10.1016/j.mib.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DJ, Minchin SD, Busby SJ. 2012. Activating transcription in bacteria. Annu Rev Microbiol 66:125–152. doi: 10.1146/annurev-micro-092611-150012. [DOI] [PubMed] [Google Scholar]
- 20.Reyes DY, Zuber P. 2008. Activation of transcription initiation by Spx: formation of transcription complex and identification of a cis-acting element required for transcriptional activation. Mol Microbiol 69:765–779. doi: 10.1111/j.1365-2958.2008.06330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajfasz JK, Martinez AR, Rivera-Ramos I, Abranches J, Koo H, Quivey RG Jr, Lemos JA. 2009. Role of Clp proteins in expression of virulence properties of Streptococcus mutans. J Bacteriol 191:2060–2068. doi: 10.1128/JB.01609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.You C, Sekowska A, Francetic O, Martin-Verstraete I, Wang Y, Danchin A. 2008. Spx mediates oxidative stress regulation of the methionine sulfoxide reductases operon in Bacillus subtilis. BMC Microbiol 8:128. doi: 10.1186/1471-2180-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin AA, Zuber P. 2012. Evidence that a single monomer of Spx can productively interact with RNA polymerase in Bacillus subtilis. J Bacteriol 194:1697–1707. doi: 10.1128/JB.06660-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano MM, Lin A, Zuber CS, Newberry KJ, Brennan RG, Zuber P. 2010. Promoter recognition by a complex of Spx and the C-terminal domain of the RNA polymerase alpha subunit. PLoS One 5:e8664. doi: 10.1371/journal.pone.0008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuber P, Chauhan S, Pilaka P, Nakano MM, Gurumoorthy S, Lin AA, Barendt SM, Chi BK, Antelmann H, Mader U. 2011. Phenotype enhancement screen of a regulatory spx mutant unveils a role for the ytpQ gene in the control of iron homeostasis. PLoS One 6:e25066. doi: 10.1371/journal.pone.0025066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker JL, Derr AM, Karuppaiah K, MacGilvray ME, Kajfasz JK, Faustoferri RC, Rivera-Ramos I, Bitoun JP, Lemos JA, Wen ZT, Quivey RG Jr. 2014. Streptococcus mutans NADH oxidase lies at the intersection of overlapping regulons controlled by oxygen and NAD+ levels. J Bacteriol 196:2166–2177. doi: 10.1128/JB.01542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng L, Das S, Burne RA. 2011. Genetic analysis of the functions and interactions of components of the LevQRST signal transduction complex of Streptococcus mutans. PLoS One 6:e17335. doi: 10.1371/journal.pone.0017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreth J, Zhang Y, Herzberg MC. 2008. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol 190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abranches J, Candella MM, Wen ZT, Baker HV, Burne RA. 2006. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. J Bacteriol 188:3748–3756. doi: 10.1128/JB.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. 1985. Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.Bryan EM, Bae T, Kleerebezem M, Dunny GM. 2000. Improved vectors for nisin-controlled expression in gram-positive bacteria. Plasmid 44:183–190. doi: 10.1006/plas.2000.1484. [DOI] [PubMed] [Google Scholar]
- 32.Seepersaud R, Needham RH, Kim CS, Jones AL. 2006. Abundance of the delta subunit of RNA polymerase is linked to the virulence of Streptococcus agalactiae. J Bacteriol 188:2096–2105. doi: 10.1128/JB.188.6.2096-2105.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barendt S, Lee H, Birch C, Nakano MM, Jones M, Zuber P. 2013. Transcriptomic and phenotypic analysis of paralogous spx gene function in Bacillus anthracis Sterne. Microbiology Open 2:695–714. doi: 10.1002/mbo3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gaballa A, Antelmann H, Hamilton CJ, Helmann JD. 2013. Regulation of Bacillus subtilis bacillithiol biosynthesis operons by Spx. Microbiology 159:2025–2035. doi: 10.1099/mic.0.070482-0. [DOI] [PubMed] [Google Scholar]
- 35.Jousselin A, Kelley WL, Barras C, Lew DP, Renzoni A. 2013. The Staphylococcus aureus thiol/oxidative stress global regulator Spx controls trfA, a gene implicated in cell wall antibiotic resistance. Antimicrob Agents Chemother 57:3283–3292. doi: 10.1128/AAC.00220-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rochat T, Nicolas P, Delumeau O, Rabatinova A, Korelusova J, Leduc A, Bessieres P, Dervyn E, Krasny L, Noirot P. 2012. Genome-wide identification of genes directly regulated by the pleiotropic transcription factor Spx in Bacillus subtilis. Nucleic Acids Res 40:9571–9583. doi: 10.1093/nar/gks755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renzoni A, Andrey DO, Jousselin A, Barras C, Monod A, Vaudaux P, Lew D, Kelley WL. 2011. Whole genome sequencing and complete genetic analysis reveals novel pathways to glycopeptide resistance in Staphylococcus aureus. PLoS One 6:e21577. doi: 10.1371/journal.pone.0021577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Fan J, Niu C, Villaruz AE, Otto M, Gao Q. 2010. Role of spx in biofilm formation of Staphylococcus epidermidis. FEMS Immunol Med Microbiol 59:152–160. doi: 10.1111/j.1574-695X.2010.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakano S, Nakano MM, Zhang Y, Leelakriangsak M, Zuber P. 2003. A regulatory protein that interferes with activator-stimulated transcription in bacteria. Proc Natl Acad Sci U S A 100:4233–4238. doi: 10.1073/pnas.0637648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakano MM, Zhu Y, Liu J, Reyes DY, Yoshikawa H, Zuber P. 2000. Mutations conferring amino acid residue substitutions in the carboxy-terminal domain of RNA polymerase alpha can suppress clpX and clpP with respect to developmentally regulated transcription in Bacillus subtilis. Mol Microbiol 37:869–884. doi: 10.1046/j.1365-2958.2000.02052.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin AA, Walthers D, Zuber P. 2013. Residue substitutions near the redox center of Bacillus subtilis Spx affect RNA polymerase interaction, redox control, and Spx-DNA contact at a conserved cis-acting element. J Bacteriol 195:3967–3978. doi: 10.1128/JB.00645-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi Y, Hulett FM. 1998. PhoP-P and RNA polymerase sigmaA holoenzyme are sufficient for transcription of Pho regulon promoters in Bacillus subtilis: PhoP-P activator sites within the coding region stimulate transcription in vitro. Mol Microbiol 28:1187–1197. doi: 10.1046/j.1365-2958.1998.00882.x. [DOI] [PubMed] [Google Scholar]