Abstract
So far the criteria for NGT and abnormal glucose tolerance (AGT) are based on HbA1c and 75 g oGTT. We present data on GV and diurnal profiles in stratified cohorts with AGT versus controls. 28 NGT, 42 AGT (15 IGT, 11 IFG, 16 CGI) matched for age and BMI classified by 75 g oGTT underwent a CGM with test meal (TM). Diurnal profiles, glucose excursion after TM, and GV (SD, MAGE) were calculated for day 2 and 3. HbA1c, with its values of 5.5 ± 0.37% versus 5.65 ± 0.36%, was within normal range. Average interstitial glucose (AiG) was 5.84 ± 0.52 mmol/l) in NGT and 6.35 ± 0.65 mmol/l in AGT (P = .002). The 2 h incremental area under curve (iAUC) from TM until 2 h after TM was 1.94 ± 1.31 mmol/l*h versus 2.89 ( ± 1.75) mmol/l*h (P = .012), AiG 2 hours after TM was 5.99 ± 1.14 mmol/l*d versus 6.64 ± 1.30 mmol/l (P = .035). Peaks of AiG after TM were 7.69 ± 1.48 mmol/l*d versus 9.18 ± 1.67 mmol/l*d (P = .001). SD was significantly higher for AGT (1.12 ± 0.37 vs. 0.85 ± 0.32 mmol/l, P = .01) and MAGE 2.26 ± 0.84 vs. 1.60 ± 0.69 mmol/l, P = .005). In this comparative analysis NGT and AGT well matched for age, BMI, and comorbidities, CGM revealed significant differences in daytime AiG, pp glucose excursion and postprandial peaks. SD and MAGE was significantly higher for subjects with AGT. I Impaired glucose homeostasis a better characterizes degree of AGTe than HbA1c and 75 g OGTT.
Keywords: glucose homeostasis, prediabetes, normal glucose tolerance, continuous glucose monitoring, glycemic variability, HbA1c
With the introduction of reliable and comfortable continuous glucose measurement systems (CGMS) it became possible to precisely investigate the glycemic variability under conditions of everyday life.1-5,10,13 So far diagnosis of abnormal glucose tolerance (AGT) or prediabetes is based on 75 g oGTT and HbA1c.6 Recently impaired glucose homeostasis and glycemic variability as early symptoms of diabetes7-10 became more and more attention. Moreover in previous studies, it was shown, that GV and pp hyperglycemia were closely related to oxidative stress,11 whereas HbA1c and average glucose levels were not.
Consequently, a number of parameters was established to describe GV and its pathology.12-15 Currently, there is an ongoing debate among experts whether increased glycemic variability with normal HbA1c may already be harmful. However most studies so far have compared cohorts of subjects/patients who are different in baseline characteristics affecting glucose tolerance and comorbidities.
In this study with matched pairs for age and BMI we analyzed differences between NGT and AGT subjects, classified by standardized 75 g oGTT. The primary question was whether parameters of GV and diurnal profiles significantly discriminate between NGT and AGT.
Methods
Eligible subjects were selected out of consecutive visitors of a health care survey in Dresden. From December 2007 to March 2009 a total of 28 subjects with normal glucose tolerance (NGT) and 42 prediabetic participants (15 with IGT, 11 participants with IFG and 16 participants with CGI) were considered. Inclusion criteria were an age between 40 and 80 years and a body-mass-index below 45 kg/m2. Exclusion criteria were preexisting diseases of the liver and kidneys, drugs affecting glucose tolerance, malignant diseases, acute infections, and the regular intake of anticoagulants. Eligible subjects were classified by 75 g standard oGTT. Participants gave informed consent. Ethic approval was obtained from the Saxon Ethic Committee. Only subjects with a complete 48-hour measurement with test meal (TM) at the morning of day 2 were included in the analysis.
CGM System
We used Minimed CGMS Gold System (Medtronic) with a seventh generation Sof sensor. During the 1-hour calibration of the device the subject was instructed first to measure capillary blood glucose using the SMBG device FreeStyle Mini and second to enter the data for calibration of plasma glucose into the CGM device. The subject’s profile was included in the study only if at least 3 measurements per day for calibration were submitted. In addition, the undisturbed CGMS measurement without any loss of data was required.
Test Meal and Recommendations for Study Protocol
Participants were instructed to continue their usual activities during this 72-hour period. They received a dietician-prepared 511-kcal standardized test meal at breakfast on the morning of the second day comprising 20 g bread, 20 g margarine, 25 g marmalade, 25 g cheese, 20 ml orange juice, and 200 ml milk mixed with banana, strawberry, or chocolate. During the remaining period, participants recorded their meals and activities and capillary glucose measurements in a standardized fashion.
Parameters of CGMS Profile
Average interstitial glucose (AiG), area under the curve (AUC), and time periods above or under different levels of interstitial glucose (iG) were calculated. Parameters to evaluate the glycemic variability were mean amplitude of glucose excursion (MAGE) and standard deviation (SD) of the arithmetic average of iG. Other parameters such as the AUC above different levels of iG, iAUC 2 hours after TM, iG peak, and ppiG 2h after TM were calculated. HbA1c and other laboratory parameters were determined in our certified laboratory.
Statistical Methods
We used SPSS 16.0 for Windows to analyze our data. To compare the different groups, we tested for normal distributions by means of the Kolmogorow–Smirnov test. If we found the tested variables normally distributed, we continued to compare arithmetic means with the Student t test for independent samples. If we didn’t find a normal distribution, data were tested with the Mann–Whitney U test. To examine correlations between nonmetric variables, we used the chi-square test. ANOVA with adjustment for AiG was used to validate the differences between groups for SD and MAGE.
Results
In this investigation we included 70 eligible subjects (46 men and 24 women). As shown in Table 1 baseline parameters of age, BMI, and sex were well balanced for both groups, with a narrow age range between 55 and 70 years. HbA1c was numerically higher only in AVG. Only HDL cholesterol was significantly lower in AGT subjects. Prevalences of comorbidities of the metabolic syndrome and cardiovascular disease were not different between groups. Eight subjects, 5 men and 3 women, were excluded, 6 with technical problems, of whom 3 showed a lack of calibration. One subject had to be excluded because of a liver enzyme ALAT of more than 2.5 to the upper reference value. One patient had to be excluded because he had already been diagnosed positively for diabetes in a prior oGTT.
Table 1.
Characteristics of Normoglycemic and Prediabetic Subjects.
| Group | NGT | AGT | P |
|---|---|---|---|
| n | 28 | 42 | |
| Female/male | 15/13 | 9/33 | |
| Age (years) | 64.21 ± 6.16 | 65.31 ± 5.79 | .452 |
| BMI (kg/m2) | 28.35 ± 3.47 | 28.87 ± 4.12 | .589 |
| Systolic blood pressure (mmHg) | 139.93 ± 17.15 | 143.60 ± 17.32 | .387 |
| Diastolic blood pressure (mmHg) | 83.43 ± 10.59 | 83.29 ± 13.48 | .963 |
| Total cholesterol (mmol/l) | 5.47 ± 0.84 | 5.02 ± 0.79 | .026 |
| Triglycerides (mmol/l) | 1.24 ± 0.81 | 1.26 ± 0.57 | .867 |
| HDL cholesterol (mmol/l) | 1.73 ± 0.31 | 1.38 ± 0.28 | <.001 |
| LDL cholesterol (mmol/l) | 3.34 ± 0.65 | 3.05 ± 0.63 | .072 |
| HbA1c (%) | 5.50 ± 0.37 | 5.65 ± 0.36 | .100 |
| PG0 diagnostic oGTT (mmol/l) | 5.36 ± 0.35 | 6.10 ± 0.58 | <.001 |
| PG120 diagnostic oGTT (mmol/l) | 5.55 ± 0.86 | 8.54 ± 1.16 | <.001 |
Values are mean ± SD.
AiG over 24 hours as well as average daytime iG were significantly higher in subjects with AGT versus NGT. The nighttime AiG however did not differ significantly (P = .087) (Table 2).
Table 2.
Differences in the Characteristics of Glucose Homeostasis and Glycemic Variability in Normoglycemic and Prediabetic Subjects.
| NGT (n = 28) |
AGT (n = 42) |
CVa
|
|||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | P | NGT | AGT |
| Mean interstitial glucose 24 hours (mmol/l) | 5.84 | 0.52 | 6.35 | 0.65 | .002 | 0.09 | 0.10 |
| Mean interstitial glucose from 6:00 am to 9:55 pm (mmol/l) | 6.07 | 0.69 | 6.62 | 0.69 | .004 | 0.11 | 0.004 |
| Mean interstitial glucose from 10:00 pm to 5:55 am (mmol/l) | 5.47 | 0.72 | 5.82 | 0.76 | .087 | 0.13 | 0.13 |
| Mean peak level of AiG (mmol/l) | 8.2 | 1.27 | 9.59 | 1.35 | .001 | 0.15 | 0.14 |
| AUC above 6.1 mmol/l ((mmol/l)*h) | 4.18 | 4.33 | 7.74 | 5.28 | .009 | 1.04 | 0.68 |
| AUC above 7.8 mmol/l ((mmol/l)*h) | 0.58 | 1.21 | 2.56 | 3.57 | .01 | 2.09 | 1.39 |
| iAUC 0-120 minutes after TM (mmol/l) | 1.94 | 1.31 | 2.89 | 1.75 | .012 | 0.68 | 0.61 |
| AiG 2 hours after TM (mmol/l) | 5.99 | 1.14 | 6.64 | 1.30 | .035 | 0.19 | 0.20 |
| AiG Peak after TM (mmol/l) | 7.69 | 1.48 | 9.18 | 1.67 | .001 | 0.19 | 0.18 |
| Time spent > 6.1 mmol/l (min) | 532 (36.9) | 359 (24.9) | 757 (52.6) | 302 (21) | .012 | 0.67 | 0.40 |
| Time spent > 6.1 mmol/l (% of 24 hours) | 36.9 | 24.9 | 52.6 | 21 | .012 | 0.67 | 0.40 |
| Time spent > 7.8 mmol/l (min) | 82 | 96 | 187 | 183 | .012 | 1.17 | 1.00 |
| Time spent > 7.8 mmol/l (% of 24 hours) | 5.7 | 6.6 | 13 | 12.7 | .012 | 1.16 | 0.98 |
| SD (mmol/l)b | 0.85 | 0.32 | 1.12 | 0.37 | .01 | 0.38 | 0.33 |
| MAGE (mmol/l)c | 1.6 | 0.69 | 2.26 | 0.84 | .005 | 0.43 | 0.37 |
CV = SD/mean.
P = .025 if adjusted for AiG with ANOVA.
P = .009 after adjustment for AiG.
In the AUC, there was a significant difference concerning glucose level above 6.1 mmol/l with 7.74 ± 5.28 mmol/l*h in subjects with AGT compared with 4.18 ± 4.33 mmol/l*h in subjects with NGT (P = .009). The AUC of glucose level above 7.8 mmol/l was different too: 2.56 ± 3.57 mmol/l*h (AGT) versus 0.58 ± 1.21 mmol/l*h (NGT) (P = .01). With respect to the time spent above 6.1 mmol/l over 24 hours a significant difference comparing AGT (757 ± 302 minutes) and NGT (532 ± 359 minutes, P = .012) was observed. Also, we found a significant difference regarding the time spent above 7.8 mmol/l with 187 ± 183 minutes in prediabetics and 82 ± 96 minutes in NGT (P = .012). There was only a small but significant difference in 2-hour post-TM glucose level (5.99 ± 1.14 versus 6.64 ± 1.3 mmol/l (P = .035). The same applies for iAUC 2 hours after the test meal. The strongest difference for test meal parameters was for peaks at 7.69 versus 9.18 mmol/l (P = .001). MAGE in AGT 2.26 ± 0.84 mmol/l was significantly higher compared to NGT versus 1.60 ± 0.69 mmol/l (P = .005). We also saw a highly significant difference in SD with 0.85 ± 0.32 mmol/l in NGT versus 1.12 ± 0.37 mmol/l in subjects with AGT (P = .01). The difference remained significant after adjustment for AiG with ANOVA: P = .025 for SD and .009 for MAGE.
As illustrated in Figure 1, subjects with AGT exhibited an impaired pp glucose regulation with the major difference between 7 and 11 Am. Of note also NGT exhibited an increase of iG levels at dawn. Only minor differences were seen at the time between 11 pm and 6 am.
Figure 1.
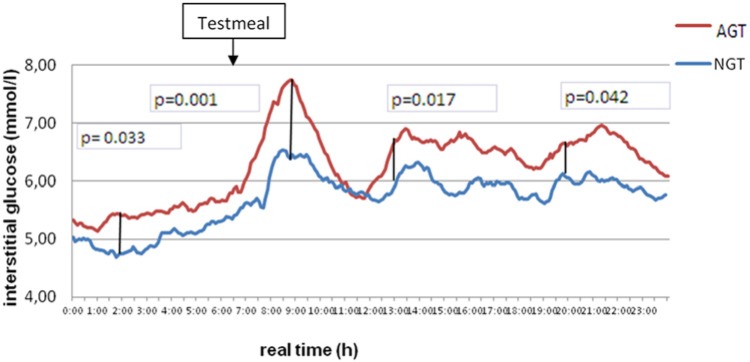
Average interstitial glucose over 24 hours in subjects with normal glucose tolerance (NGT) and abnormal glucose tolerance (AGT) at day 2 of measurement.
Discussion
Our study compares for the first time discriminative power of different metrics of glucose homeostasis calculated from CGM in age-, BMI-, and sex-matched individuals with NGT and AGT. Reference populations were also well matched for comorbidities and other conditions affecting glucose tolerance. To better control for postprandial glucose regulation and MAGE we introduced a standardized TM at day 2. We saw minor but significant differences for AiG at daytime, 2-hours postmeal iG, and glucose increment 2 hours after TM. Highly significant differences were observed of peak values after TM and AUC above 6.1 mmol/l. Furthermore, established parameters of glycemic variability, SD and MAGE, were significantly higher in AVG compared to NGT: 1.12 ± 0.37 versus 0.85 ± 0.32 mmol/l, P = .01 and 2.26 ± 0.84 versus 1.6 ± 0.69 mmol/l (P = .005). Hill et al reported a normal reference range in 70 subjects with fasting plasma glucose of < 6.7 mmol/l for MAGE of 0.0-3.5 mmol/l.15 Normal reference ranges for glycemic variability in 434 healthy Chinese subjects were published in 2011 by Zhou et al.14 For these people at an age between 20 and 69 years with a BMI of 21.8 ± 1.7 kg/m2 the 95th percentile of MAGE and SD were 3.86 and 1.4, respectively.
The discriminative power of these dynamic indices could be shown for the first time by Costa et al.7 They depicted significant differences between normoglycemic and prediabetic subjects concerning the time spent above 7.8 mmol/l and the time spent between 4 and 7.8 mmol/l. Compared to HbA1c and FPG, 2 hours postprandial glucose, SD, and MAGE proved to be the more sensitive parameters to reflect an impaired glucose homeostasis and risk of hypoglycemia.8,9
Interestingly, we found that an increase in MAGE and SD was associated with an extended time spent in hyperglycemic areas of interstitial glucose, while the time spent at low glucose levels was decreased. As with CV after adjustment of AiG for both groups SD and MAGE were significantly higher in people with AVG with confirmed HbA1c still in the range of matched people with NGT.
The question however remains open whether this is clinically relevant. The increase in MAGE and SD in the prediabetic group at a normal HbA1c level may be an explanation for the higher cardiovascular risk in prediabetes. As shown by Monnier and colleagues in patients with advanced type 2 diabetes MAGE and postprandial hyperglycemia are closely correlated to oxidative stress whereas HbA1c and average glucose levels were not.11 In the Risk Factors in IGT for Atherosclerosis and Diabetes (RIAD) study pp hyperglycemia but neither fasting plasma glucose nor HbA1c was significantly associated with intima media thickness of common carotid arteries.13,16
In a large observational trial, the DECODE study in subjects with impaired glucose postprandial hyperglycemia in multivariate analysis was a significant predictor of cardiovascular complications and all cause mortality.17 In the future, integrated models of parameters of GV with HbA1c, FPG, and PPG18 may improve the risk prediction. The approach of such a model is the glucose pentagon model, which combines the generally accepted HbA1c with important parameters for glycemic variability, gained by CGM.19
So far no evidence-based data with cardiovascular outcome and CGMS are available to confirm Monnier et al’s hypothesis.
Conclusion
Diagnostic parameters of glycemic variability such as SD, MAGE, and postprandial glucose excursion measured with CGMS were significantly different between subjects with NGT and prediabetes well matched for age and BMI and within a normal range for HbA1c. Thus abnormalities in glucose homeostasis precede an increase in HbA1c.
Weaknesses of Our Study
We compared only small cohorts of subjects with a 2-day recording of CGMS with an older device. However, subjects were well characterized and matched. Furthermore, no surrogate parameters of cardiovascular risk were measured to be correlated to CGMS metrics. Neither did we directly measure ß-cell function.
Large-scale prospective studies with overt diabetes and cardiovascular events and microvessel disease as endpoints are needed to validate CGM-derived metrics.
Footnotes
Abbreviations: AGT, abnormal glucose tolerance; AiG, average interstitial glucose; AUC, area under curve; BMI, body mass index; CGM, continuous glucose monitoring; CGT, combined glucose intolerance; FPG, fasting plasma glucose; GV, glycemic variability; iAUC, incremental area under the curve; IFG, impaired fasting glucose; iG, interstitial glucose; IGT, impaired glucose tolerance; MAGE, mean amplitude of glucose excursions; NGT, normal glucose tolerance; oGTT, oral glucose tolerance test; PG0, plasma glucose to start of oGTT; PG120, plasma glucose 120 min after oGTT; ppiG 2h, post prandial interstitial glucose 2 hours after test meal; SD, standard deviation; TM, test meal.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Andreas Thomas is employee of Medtronic GmbH.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36(suppl 1):S11-S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International Diabetes Federation. Global Guidelines for Type 2 Diabetes. www.idf.org. Accessed July 26, 2013.
- 3. Klonoff DC. Continuous glucose monitoring roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28(5):1231-1239. [DOI] [PubMed] [Google Scholar]
- 4. Gross TM, Bode BW, Einhorn D, et al. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol Ther. 2000;2(1):49-56. [DOI] [PubMed] [Google Scholar]
- 5. Gross TM, Mastrototaro JJ. Efficacy and reliability of the continuous glucose monitoring system. Diabetes Technol Ther. 2000;2(1):19-26. [DOI] [PubMed] [Google Scholar]
- 6. Alqahtani N, Khan WA, Alhumaidi MH, Ahmed YA. Use of glycated hemoglobin in the diagnosis of diabetes mellitus and pre-diabetes and role of fasting plasma glucose, oral glucose tolerance test. Int J Prev Med. 2013;4(9):1025-1029. [PMC free article] [PubMed] [Google Scholar]
- 7. Costa B, Vizcaino J, Pinol JL, Cabre JJ, Fuentes CM, Record Research Group. Relevance of casual undetected hyperglycemia among high-risk individuals for developing diabetes. Diabetes Res Clin Pract. 2007;78(2):289-292. [DOI] [PubMed] [Google Scholar]
- 8. Engler B, Koehler C, Hoffmann C, et al. Relationship between HbA1c on target, risk of silent hypoglycemia and glycemic variability in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119(1):59-61. [DOI] [PubMed] [Google Scholar]
- 9. Hanefeld M, Koehler C, Hoffmann C, Wilhelm K, Kamke W, Gerstein H. Effect of targeting normal fasting glucose levels with basal insulin glargine on glycaemic variability and risk of hypoglycaemia: a randomized, controlled study in patients with early Type 2 diabetes. Diabet Med. 2010;27(2):175-180. [DOI] [PubMed] [Google Scholar]
- 10. Mazze RS, Strock E, Wesley D, et al. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther. 2008;10(3):149-159. [DOI] [PubMed] [Google Scholar]
- 11. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681-1687. [DOI] [PubMed] [Google Scholar]
- 12. Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose of HbA1c level. Diabetes Care. 2000;23(12):1830-1834. [DOI] [PubMed] [Google Scholar]
- 13. Marling CR, Struble NW, Bunescu RC, Shubrook JH, Schwartz FL. A consensus perceived glycemic variability metric. J Diabetes Sci Technol. 2013;7(4):871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou J, Li H, Ran X, et al. Establishment of normal reference ranges for glycemic variability in Chinese subjects using continuous glucose monitoring. Med Sci Monit. 2011;17(1):CR9-CR13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13(9):921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanefeld M, Koehler C, Henkel E, Fuecker K, Schaper F, Temelkova-Kurtschiev TS. Post-challenge hyperglycaemia relates more strongly than fasting hyperglycaemia with carotid intima-media thickness: the RIAD Study. Risk factors in impaired glucose tolerance for atherosclerosis and diabetes. Diabet Med. 2000;17(12):835-840. [DOI] [PubMed] [Google Scholar]
- 17. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE Study Group. European Diabetes Epidemiology Group. Diabetes epidemiology: collaborative analysis of diagnostic criteria in Europe. Lancet. 1999;354(9179):617-621. [PubMed] [Google Scholar]
- 18. Monnier L, Colette C. Glycemic variability: should we and can we prevent it. Diabetes Care. 2008;31(suppl 2):S150-S154. [DOI] [PubMed] [Google Scholar]
- 19. Thomas A, Schönauer M, Achermann F, et al. The “glucose pentagon”: assessing glycemic control of patients with diabetes mellitus by a model integrating different parameters from glucose profiles. Diabetes Technol Ther. 2009;11(6):399-409. [DOI] [PubMed] [Google Scholar]


