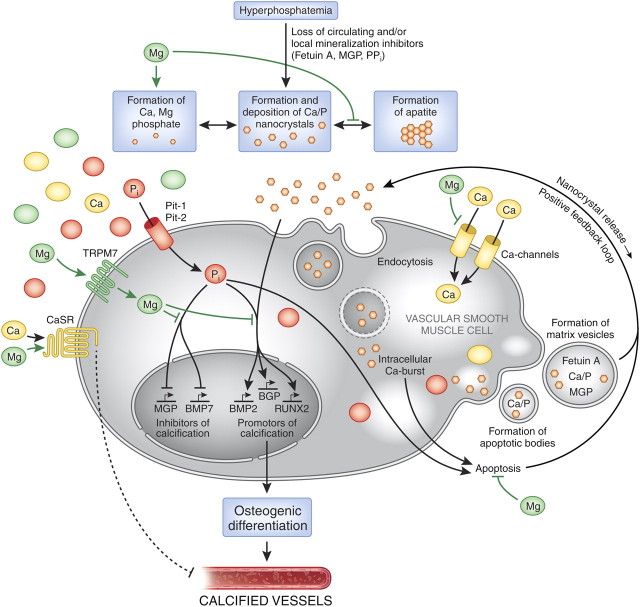
Fig. 3.
The putative protective roles of magnesium in the course of vascular calcification. Abnormalities in mineral metabolism, particularly hyperphosphataemia as well as the loss of circulating and/or local mineralization inhibitors such as fetuin A, MGP or PPi, initially lead to the formation and deposition of Ca/P nanocrystals [25, 26]. These nanocrystals are taken up by VSMCs, most likely via endocytosis [29]. The lysosomal degradation of the endocytosed crystals results in an intracellular release of calcium and phosphate. Inorganic phosphate additionally accumulates in the cell via uptake through the sodium-dependent phosphate transporter Pit-1 (and Pit-2) [30, 31]. In an attempt to compensate for excess Ca/P, VSMCs form matrix vesicles loaded with Ca/P products as well as the mineralization inhibitors fetuin A and MGP [32]. The intracellular Ca-burst induced by endocytosed nanocrystals [33] as well as the phosphate uptake [34] trigger VSMC apoptosis, resulting in the formation of Ca/P containing apoptotic bodies [35]. Both apoptotic bodies and matrix vesicles are ultimately causing a positive feedback loop through nanocrystal release into the surrounding milieu, thus amplifying the calcification process. Furthermore, Ca/P nanocrystals as well as Pi induce the expression of genes that promote the calcification/mineralization process such as RUNX2, BMP2 and BGP, while at the same time repressing the expression of MGP or BMP7, factors that are known to inhibit the progression of calcification. This causes a transdifferentiation of VSMCs to osteoblast-like cells, ultimately resulting in vessel calcification. Magnesium interferes with this process of vascular calcification on different levels: firstly, Mg inhibits the transformation from amorphous Ca/P to any apatite (carbonatohydroxyapatite) [36, 37] and forms Mg-substituted tricalcium (whitlockite) under certain conditions, which is more soluble than apatite [37], resulting in smaller, more soluble deposits [37, 38]. Secondly, magnesium functions as a Ca-channel antagonist [39] and thus inhibits the entry of Ca into the cells. Thirdly, within the cell, via TRPM7, Mg restores the balance between the expression of calcification promotors and inhibitors by neutralizing the inhibition of MGP and BMP7 induced by phosphate [40]. Furthermore, it regresses the phosphate- and Ca/P nanocrystal-induced enhanced expression of RUNX2 and BMP2 [41] preventing the VSMCs from osteoblastic conversion and calcification. In addition, magnesium acts on the CaSR [42]; activation of this receptor by calcimimetics has been shown to inhibit VSMC calcification [43]. The underlying molecular mechanisms have not been identified so far. BGP, bone GLA protein, osteocalcin; BMP, bone morphogenetic protein; Ca, calcium; CaSR, calcium-sensing receptor; Mg, magnesium; MGP, matrix Gla protein; Pi, inorganic phosphate; Pit, inorganic phosphate transporter; PPi, inorganic pyrophosphate; RUNX2, runt-related transcription factor 2, cbfa1, core-binding factor subunit alpha-1; TRPM, transient receptor potential melastatin; VSMC, vascular smooth muscle cell.