Abstract
We investigated the effect of the expanded criteria donor (ECD) label on (i) recovery of kidneys and (ii) acceptance for transplantation given recovery. An ECD is age ≥ 60, or age 50–59 with ≥ 2 of 3 specified comorbidities. Using data from the Scientific Registry of Transplant Recipients from 1999 to 2005, we modeled recovery rates through linear regression and transplantation probabilities via logistic regression, focusing on organs from donors just-younger versus just-older than the ECD age thresholds. We split the sample at July 1, 2002 to determine how decisions changed at the approximate time of implementation of the ECD definition. Before July 2002, the number of recovered kidneys with 0-1 comorbidities dropped at age 60, but transplantation probabilities given recovery did not. After July 2002, the number of recovered kidneys with 0-1 comorbidities rose at age 60, but transplantation probabilities contingent on recovery declined. No similar trends were observed at donor age 50 among donors with ≥ 2 comorbidities. Overall, implementation of the ECD definition coincided with a reversal of an apparent reluctance to recover kidneys from donors over age 59, but increased selectiveness on the part of surgeons/centers with respect to these kidneys.
Introduction
The persistent shortage of organs available for transplantation is one of most vexing policy challenges facing the United States’ health care system. On December 31, 2008, 80,972 patients were wait-listed for a kidney transplant, up from 75,834 just a year earlier (1). The median time on the kidney waiting list exceeded three years (2), and the annual mortality rate while on the waiting list was 7 percent (1). Responses to organ shortages in the United States and other countries include increased use of organs from older donors (3), public education efforts to raise the number of donations of cadaveric organs (4), measurement of the performance of the organ procurement system in hopes of improving organ acquisition rates (5), increased numbers of living kidney donations (6), and consideration of financial incentives for donation (7, 8).
Given this increase, a formal definition of an expanded criteria donor (ECD) for kidneys was published in 2002 (9), and a separate wait-listing procedure was developed for patients willing to consider accepting an ECD [vs. a standard criteria donor (SCD)]. ECDs are defined as deceased donors that are 1) aged 60 or older, regardless of the presence or absence of comorbid conditions, or 2) aged 50–59 with at least two of three specified comorbid conditions (history of hypertension, elevated serum creatinine indicating suboptimal donor kidney function (>1.5 mg/dl), death caused by cerebrovascular accident). The increased use of organs from older donors has been a particularly significant shift in clinical policy. The number of kidneys transplanted from deceased donors meeting the ECD criteria rose by 50 percent between 1997 and 2006 compared to an SCD increase of only 22.3 percent.
Transplantation of organs from older donors reduces average time on the waiting list, but results in higher rates of transplant graft failure than would arise with the use of kidneys from younger donors. In defining an ECD, Port et al (9) used a graft failure hazard ratio for ECD kidneys relative to an ideal donor (age < 40 with none of the three comorbid conditions) of at least 1.70. Coincidentally, the relative risk of graft failure for ECD transplants compared to SCD kidneys (all those that do not meet ECD criteria) was 1.69. A recent analysis has shown favorable selection from the pool of potential ECDs for transplantation, estimating that the hazard ratio would rise to 2.20 if all ECDs were accepted for transplantation (10). Therefore, understanding how kidneys from within the ECD designation are selected for transplant is crucial to evaluating the tradeoffs between waiting time and graft survival. If the selection process is too stringent, or insufficiently stringent, the use of the ECD pool would be suboptimal.
In this regard, deceased donor kidney transplantation can be viewed as the outcome of two sequential decision processes. The first process is completed by organ procurement organizations (OPOs), the 58 entities contracted by the federal government to manage the organ donation and recovery process in all hospitals within their defined service areas. OPOs work with hospitals to identify potential donors, gain consent for donation from next-of-kin, and arrange for the recovery and allocation of donated organs. The second process is the acceptance of an organ offered to a candidate at a particular transplant center. Transplant centers may vary in their willingness to use ECDs. For example, center practices with respect to ECD listing have been documented. More than a quarter of centers listed fewer than 20 percent of their transplant candidates for ECDs, while a quarter of centers listed more than 90 percent of their transplant candidates for ECDs (11).
It has been noted that the dichotomous distinction between SCD and ECD kidneys may be interpreted as distinction between “good” and “bad” organs that may influence procurement and acceptance decisions (12). Therefore, our objective was to evaluate the association between the ECD label and deceased donor kidney recovery rates and the probability of transplantation of such kidneys once recovered. To do so, we compared recovery and transplantation decisions for donors barely younger and barely older than the ECD thresholds (i.e., ages 50 and 60). Recent results from Rao et al (13) show that graft failure rates exhibit a distinct V-shaped relationship with age. Specifically, graft failure rates decrease steadily with age up to age 18, then increase steadily above age 18. There is no large, discrete drop in transplant graft outcomes at either donor age 50 or 60. Therefore, if a discrete change in the recovery or transplant rates at either of the ECD threshold ages (50 and 60) were found, it would appear reasonable to conclude that the ECD label itself influences decisions about which kidneys to recover and which to transplant. Conversely, the lack of a discrete change at either of the age thresholds would be consistent with each kidney being evaluated on its own merits and without regard to the ECD label per se. Sung and colleagues (3) previously established an increase in ECD kidney recoveries and transplants following the ECD policy implementation. Our analysis complements theirs by separately testing for explicit breaks in the recovery and transplantation rates before and after mid-2002 for donors “near” the ECD cutoffs.
Methods
We used national data from the Scientific Registry of Transplant Recipients (SRTR) on all recovered deceased donor kidneys and all deceased donor kidney transplants from January 1, 1999 to December 31, 2005. Since the analysis was focused on evaluation of the ECD age thresholds, the study population consisted of kidneys from donors aged 54 to 65 with 0-1 of the listed comorbidities, and donors aged 44–55 with 2-3 of the listed comorbidities.
Kidneys recovered for transplantation were categorized by donor age (years), and linear regression was used to model the number of recoveries (serving as the response variable) as a function of donor age (covariate). This analysis is unadjusted, in the sense that donor age is the only covariate in the model. Data were not available on the number of kidneys that could have been recovered, because this population cannot be defined using currently available data. Therefore, we cannot model the percent of potential kidneys recovered, and our estimates based on actual number of donors may be influenced by unobservable fluctuations in the size of the potential donor pool by age and time period.
Transplantation probability, conditional on recovery, was modeled using logistic regression. This component of the analysis utilized all recovered kidneys, with transplantation serving as the binary (yes/no) response in the logistic model. Adjustment covariates included donor demographics (sex, race), cause of death (cerebrovascular accident, anoxia, tumor, other), lifestyle habits (alcohol, smoking, cocaine, other drug use), disease history (cancer, diabetes, hypertension), serology results (hepatitis B antibody, hepatitis C antibody, cytomegalovirus antibody), cardiovascular disease, high creatinine level, blood urea nitrogen, presence of protein in urine, clinical infection, and having tattoos.
In the subgroup of donors with none or one of the three previously listed ECD comorbid conditions, donor ages were grouped as 54–59, 60, and 61–65. In this subgroup, the 54–59 year-olds would not be classified as ECD, while donors aged 60–65 would meet the ECD criteria. Similarly, among donors with two or three of the ECD comorbid conditions, ages were grouped into 44–49, 50, and 51–55, where donors aged 50–55 would be classified as ECD, unlike those below age 50.
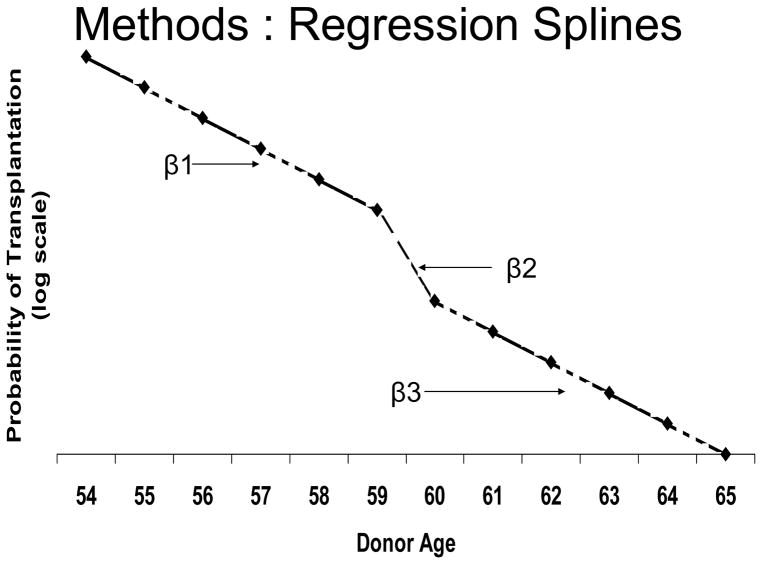
With respect to the evaluation of the ECD labeling effect, the models for recovery frequency (linear regression) and the probability of transplant given recovery (logistic model) used linear splines, as depicted generically in Figure 1. The effect of age was fitted as piece-wise linear with knots chosen to bracket the ECD age threshold. For example, as indicated in Figure 1, for donors age 54–65, the regression line was allowed to change slope at ages 59 and 60. To illustrate the interpretation of the coefficients, suppose they follow the pattern in Figure 1. β1 is negative and represents the decline in the probability of transplantation per year of age as age rises from 54 to 59. The increase in the absolute value of the slope between ages 59 and 60 (β2 > β1 in absolute value) implies that the downward trend in transplantation probability by age seen for ages 54–59 is discretely accentuated at age 60. Had there not been this break in the trend at age 60, it would have been the case that β2 = β1, reflecting a continuation of the earlier trend line. As drawn in Figure 1, after age 60, the trend by age returns to the level that prevailed below age 60 (β3 = β1). However, due to the break that occurred at age 60, the transplantation probability at ages over 60 has shifted down relative to what it would have been had the break had not occurred. In other words, the reduction at age 60 persists at older ages even though the age trend (slope) returns to the same level seen at ages 54–59 (the line at ages over 60 is parallel to, but lower than, the line at ages 54–59). In this way, we tested for the existence of a sudden drop at age 60, then evaluated whether the drop at age 60 persisted across ages 61–65. We adopted a similar strategy for the donors aged 44–55, for which the spline had breaks at age 49 and 50.
Figure 1.
Illustration of spline pattern expected if the relationship between donor age and probability of transplantation changes at the age used to define ECD status.
We grouped the data into two calendar periods: January 1, 1999 to June 30, 2002 (just prior to the development of the ECD definition) and July 1, 2002 to December 31, 2005 (just following the ECD definition). Threshold-related phenomena observed during the post-ECD period would be viewed with skepticism if they were also observed during the pre-ECD period. The ECD policy did not formally take effect until November 1, 2002. However, we used a slightly earlier cut point (at the mid-point of our time interval) to allow for the possibility that any behavior changes may have already begun to occur in anticipation of the policy. In particular, the expanded criteria issue was one focus of a prominent consensus meeting held on March 28–29, 2001, and a report on that meeting was published in September 2002 (14). Given that the difference between the mid-point of our time interval and formal implementation of the ECD policy was only four months out of a seven year time window, it is likely that the results would have been quite similar using a slightly different cut point. Finally, as a natural follow-up to the recovery frequency and transplant probability analyses, we estimated the shortfall/excess of kidneys due to changing recovery or transplant patterns at the ECD threshold. Specifically, the shortfall/excess was calculated as the actual kidney numbers (recoveries and transplants) minus the predicted kidney numbers (recoveries and transplants) in a hypothetical scenario where the trend before 60 continues after 60.
Statistical analyses were performed with SAS software, version 9.1 (SAS Institute; Cary, North Carolina, USA). The authors have followed the suggestions of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines for reporting observational studies.
Results
A summary of the donor kidneys used in the analysis is listed in Table 1. Approximately two-thirds of the 15,708 recovered kidneys were transplanted.
Table 1.
Percentage of Donor Kidneys Transplanted Among Those Recovered
| Donor Characteristics | Recovered | Transplanted | Percent Transplanted |
|---|---|---|---|
| Age 44–55, with 2-3 comorbid conditions | 8,728 | 5,387 | 62% |
| Age 54–65, with 0-1 comorbid conditions | 6,980 | 5,253 | 75% |
| Total | 15,708 | 10,640 | 68% |
As stated previously, the analysis evaluated referral frequency and transplant probability (given referral) for two groups: (i) donors age 44–55 with two or three ECD comorbid conditions and (ii) donors age 54–65 with zero or one comorbidity. In group (i), donors age ≥ 50 would be classified as ECD, while those age 44–49 would be considered non-ECD. Similarly, in group (ii), only donors age ≥ 60 would meet the ECD criteria. We evaluated trends for each of these two groups separately during the pre- and post-ECD periods.
No noteworthy trends were observed for donors age 44–55 with two or three of the listed comorbidities (data not shown). As a result, we focused on the age 54–65 group, for which some notable findings were observed.
Trends in the number of kidneys recovered are described in Table 2, for donors age 54–65 with zero or one of the ECD comorbid conditions. Note that the values in Table 2 line up with the model depicted in Figure 1. From ages 54–59, the number of kidneys recovered decreased significantly (p=0.004) with age for both the pre-ECD (January 1999 to June 2002; p=0.004) and post-ECD (July 2002 to December 2005; p<0.0001) eras. For the pre-ECD period, the slope (rate of change in the recovery rate per year increase in age) was not significantly different for age 60 or age 61–65, than for the 54–59 age group. For the post-ECD period, a near-significant increase (p=0.06) was observed in the procurement rate for age 60 compared to that for age 54–59.
Table 2.
Trends in Number of Kidneys Recovered For Transplantation: Donors Age 54–65 With 0-1 Comorbid Conditions
| 01/01/1999–06/30/2002 | 01/01/1999–06/30/2002 | 07/01/2002–12/31/2005 | 07/01/2002–12/31/2005 | |
|---|---|---|---|---|
| Trend* | Estimate | p | Estimate | p |
| Slope**(β1): age 54–59 | −16.54 | 0.004 | −39.63 | <0.0001 |
| Difference in slopes (β2-β1):age 54–59 vs. age 59–60 | −36.12 | 0.12 | 58.06 | 0.06 |
| Difference in slopes (β3-β2):age 60–65 vs. age 59–60 | 36.61 | 0.11 | −46.37 | 0.12 |
| Difference in slopes (β3-β1):age 60–65 vs. age 54–59 | 0.49 | 0.26 | 11.69 | 0.10 |
Trends were estimated using a linear regression model featuring linear splines.
Slopes are interpreted as the covariate-adjusted change in number of donor kidneys recovered per 1-year increase in donor age.
All β labels refer to Figure 1
In Table 3, trends in the probability of a recovered kidney being transplanted are shown for donors age 54–65 with zero or one of the ECD comorbid conditions. The trends quantified in Table 3 follow the model pictured in Figure 1. For donors age 54–59 (i.e., who would not meet the ECD criteria), transplant probability decreased significantly with increasing age in the pre-ECD era (p=0.009), but not in the post-ECD era (p=0.44). Compared to the slope across age 54–59, the slope at age 60 decreased significantly during the post-ECD era (p=0.001). The odds of transplantation (given recovery) decreased by 8.5% per year increase in age for the 54–59 age group. However, the drop in transplant odds at age 60, relative to age 59, was 44% (OR=0.56). In contrast, during the pre-ECD period, there was no sudden drop in transplant probability at age 60, nor was the trend significantly different across the 54–59, 60, and 61–65 age ranges.
Table 3.
Trends in Odds of Transplantation Among Recovered Kidneys: Donors Age 55–64 With 0-1 Comorbid Conditions, Before and After ECD Policy Change
| 01/01/1999–06/30/2002 | 01/01/1999–06/30/2002 | 07/01/2002–12/31/2005 | 07/01/2002–12/31/2005 | |
|---|---|---|---|---|
| Trend* | Odds Ratio | p | Odds Ratio | P |
| Slope**(β1): age 54–59 | 0.915 | 0.009 | 0.973 | 0.442 |
| Difference in slopes: age 54–59 vs. age 59–60 | 0.997 | 0.986 | 0.557 | 0.001 |
| Difference in slopes: age 60–65 vs. age 59–60 | 0.890 | 0.509 | 1.629 | 0.007 |
| Difference in slopes: age 60–65 vs. age 54–59 | 0.887 | 0.062 | 0.907 | 0.002 |
Trends were estimated using a logistic regression model featuring linear splines.
Slopes are interpreted as the covariate-adjusted change in the odds*** of transplantation (among recovered kidneys) per 1-year increase in donor age.
odds = probability (transplant)/{1-probability (transplant)}, meaning that as the odds increases (decreases), the probability also increases (decreases)
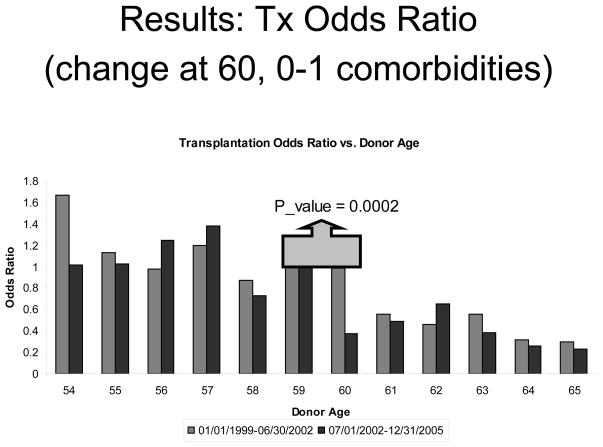
Figure 2 displays the results of a logistic regression model for likelihood of transplantation within the 54–65 age group. For this model, we coded donor age as a categorical covariate, with age 59 serving as the reference (OR=1.0), so that every age is compared to age 59. Of primary interest here is the difference between the pre- and post-ECD periods with respect to the age 60 vs. 59 comparison. As shown in Figure 2, the difference in transplant odds between age 59 and 60 was modest during the pre-ECD era (−2%; OR=0.98), but was very strong during the post-ECD period (−62%; OR=0.38). The difference in these trends easily reached statistical significance (p=0.0002). Although single year trends are subject to noise, the Figure also reflects a drop going from age 60 to age 61 in the pre-ECD period. However, a fairly consistent pattern emerges when comparing single year transplant odds in the pre- and post-ECD periods, with lower transplantation odds in the post-ECD period for five of the six ages at or above 60 years.
Figure 2.
Results of a logistic regression model for transplantation probability within the 54–65 donor age group with 0 or 1 comorbidity. 23
As shown in Table 4, we estimated that the lower rate of recovery pre-July 1, 2002 resulted in approximately 200 fewer available kidneys and transplants than would have occurred had the trends for donor ages 54–59 continued for ages 60–65. Conversely, the increase in recovery post-July 1, 2002 resulted in approximately 500 more recovered kidneys than would have occurred had trends for donor ages 54–59 continued for ages 60–65, but only 200 additional transplants due to the decline in transplantation probabilities contingent on recovery. No similar changes in trends were observed at donor age 50 for those with two or three of the ECD comorbidities.
Table 4.
Age-Specific Difference (i.e., Shortfall or Excess)* in Number of Kidneys Recovered and Transplanted: Before Versus After the Establishment of the ECD Definition
| 60 | 61 | 62 | 63 | 64 | 65 | Total | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 1999–Mid-2002 | Recovered | −51 | −4 | −62 | −20 | −38 | −35 | −209 |
| Transplanted | −33 | −17 | −57 | −11 | −46 | −38 | −202 | |
|
| ||||||||
| Mid-2002–2005 | Recovered | 35 | 80 | 98 | 116 | 82 | 112 | 523 |
| Transplanted | −19 | 29 | 68 | 60 | 35 | 56 | 229 | |
Negative sign indicates shortfall; positive sign indicates excess 22
Discussion
Age > 60 and the ECD label both appear to influence decision-makers, but the behavioral patterns seem to have shifted substantially in 2002. Implementation of the ECD definition coincided with a reversal of an apparent reluctance to recover kidneys from donors over age 59, but was accompanied by greater selectivity on the part of surgeons with respect to transplanting these kidneys. These findings are only associations rather than conclusive proof of causation. If other factors specific to organs from donors 60 years and older changed at the same time the ECD definition was formalized, the observed patterns might have been seen even if there was no causal impact of the ECD label itself. For example, reporting or publication of favorable outcomes from single centers combined with the continuing increase in waiting time for an SCD kidney could have increased the pressure to transplant “marginal” organs concurrently with the implementation of the ECD criteria. Likewise, activities such as the Organ Donation Breakthrough Collaborative may have affected trends over our study period.
Before July 1, 2002, OPOs appeared less likely than expected (given trends in recoveries among donors aged 54–59) to recover donors over 60, but centers/surgeons did not seem less likely than expected to transplant such kidneys once recovered. This pattern is consistent with OPOs pre-selecting only the most promising kidneys for transplantation in this donor age group. The reduction in recoveries (relative to the expected number if trends based on donors aged 54–59 are projected onto the age 60–65 donor cohorts) translates to approximately a one-to-one ratio of a decline in actual transplants.
Post-June 30, 2002, OPOs appear to have become more liberal about recovering ECDs 60 and older, but surgeons/centers appear to have responded by becoming more selective about transplanting the kidneys recovered from these donors. The extra recoveries do appear to have resulted in extra transplants, but the number of transplants increased by only about 40% as much as the number of recoveries increased (both relative to the expected number if trends based on donors aged 54–59 are projected onto the age 60–65 donor cohorts).
The lack of apparent behavior changes at the age 50 cutoff for ECDs with two or more of the listed comorbidities is intriguing. This may reflect a general perception that age 55 or 60 is the rough cutoff for a “marginal” donor (15). Therefore, the formalization of the ECD definition may have been more likely to affect behavior at that margin than at the age 50 margin.
As more experience with ECDs accrues, it will be useful to ascertain whether these changes in behavior persist. In addition, future research could attempt to establish whether or not patterns of ECD recovery and acceptance for transplant vary across OPOs, transplant centers, or regions of the country. These findings also suggest that it may be moving to a more continuous description of donor or organ quality, rather the a dichomous ECD vs. SCD description, may be useful. Clearly, transplants from ECDs represent only a partial response to the long-standing organ shortage. However, their role in providing access to kidney transplants for substantial numbers of patients on the waiting list has been established, and further research can help optimize their use.
Acknowledgments
Funding Sources: The Scientific Registry of Transplant Recipients is funded by contract number 234-2005-37009C from the Health Resources and Services Administration, U.S. Department of Health and Human Services. The views expressed herein are those of the authors and not necessarily those of the U.S. Government.
Footnotes
Human Subjects Statement: This study was approved by HRSA’s SRTR project officer. HRSA has determined that this study satisfies the criteria for the IRB exemption described in the “Public Benefit and Service Program” provisions of 45 CFR 46.101(b)(5) and HRSA Circular 03.
References
- 1.Scientific Registry of Transplant Recipients. [accessed on August 19, 2009];Program-Specific Reports. Available at: http://www.ustransplant.org/csr/current/nationalViewer.aspx?o=KI.
- 2.National Kidney Foundation. [accessed on May 5, 2008];A to Z Health Guide. Available at: http://www.kidney.org/atoz/atozItem.cfm?id=114.
- 3.Sung RS, Guidinger MK, Lake CD, McBride MA, Greenstein SM, Delmonico FL, et al. Impact of the expanded criteria donor allocation system on the use of expanded criteria donor kidneys. Transplantation. 2005;79(9):1257–1261. doi: 10.1097/01.tp.0000161225.89368.81. [DOI] [PubMed] [Google Scholar]
- 4.Persijn GG, van Netten AR. Public education and organ donation. Transplantation Proceedings. 1997;29:1614–1617. doi: 10.1016/s0041-1345(97)82536-5. [DOI] [PubMed] [Google Scholar]
- 5.Stogis S, Hirth RA, Strawderman RL, Banaszak-Holl J, Smith DG. Using a Standardized Donation Ratio to Assess the Performance of Organ Procurement Organizations. Health Services Research. 2002;37:1329–1344. doi: 10.1111/1475-6773.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scientific Registry of Transplant Recipients. [accessed on May 5, 2008];Program-Specific-Reports. Available at: http://www.ustransplant.org/csr/current/FastFacts/datatour.aspx?s=6.
- 7.Arnold R, Bartlett S, James Bernat, Colonna J, Dafoe D, Nancy Dubler, et al. Financial incentives for cadaver organ donation: an ethical reappraisal. Transplantation. 2002;73(8):1361–1367. doi: 10.1097/00007890-200204270-00034. [DOI] [PubMed] [Google Scholar]
- 8.Byrne MM, Thompson P. A positive analysis of financial incentives for cadaveric organ transplantation. Journal of Health Economics. 2001;20:69–83. doi: 10.1016/s0167-6296(00)00065-5. [DOI] [PubMed] [Google Scholar]
- 9.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, et al. Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation. 2002;74(9):1281–1286. doi: 10.1097/00007890-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 10.Pan Q, Schaubel DE. Proportional hazards regression based on biased samples and estimated selection probabilities. Canadian Journal of Statistics. 2008;36(1):111–127. [Google Scholar]
- 11.Schold JD, Howard RJ, Scicchitano MJ, Meier-Kriesche HU. The expanded criteria donor policy: An evaluation of program objectives and indirect ramifications. Am J Transplant. 2006;6 (7):1689–1695. doi: 10.1111/j.1600-6143.2006.01390.x. [DOI] [PubMed] [Google Scholar]
- 12.Freeman RB, Klintmalm GB. It is time to re-think the ‘Extended Criteria’. Am J Transplant. 2006;6:2225–2227. doi: 10.1111/j.1600-6143.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- 13.Rao PS, Guidinger MK, Andreoni KA, Christensen LL, Jia X, Merion RM, et al. Kidney donor risk index [abstract] Am J Transplant. 2007;7(suppl 2):307. [Google Scholar]
- 14.Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2:701–711. doi: 10.1034/j.1600-6143.2002.20804.x. [DOI] [PubMed] [Google Scholar]
- 15.Alfrey EJ, Lu AD, Carter JT, Dafoe DC. Matching does not improve outcome from aged marginal kidney donors. Transplantation Proceedings. 2001;33(1):1162–1163. doi: 10.1016/s0041-1345(00)02443-x. [DOI] [PubMed] [Google Scholar]