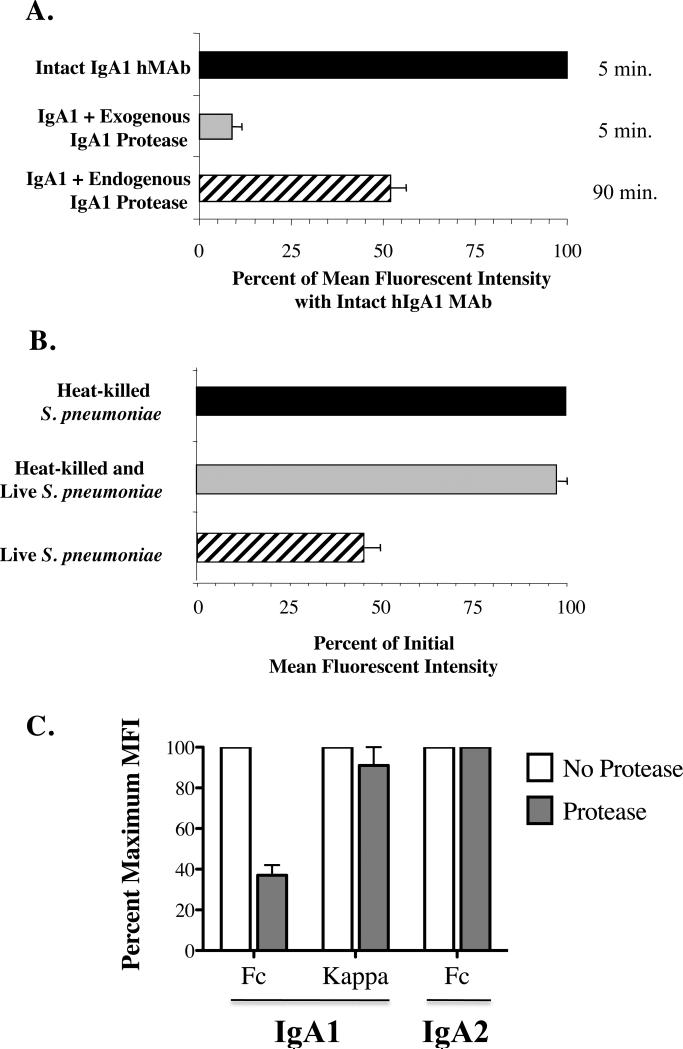
Figure 2. IgA1 protease reduces IgA1 Fcα bound to S. pneumoniae.
Detection of serotype-specific human IgA1 hMAb bound to serotype 2 organisms (P210). In Fig. 2A, organisms were exposed on ice for 30 minutes to intact hMAb (black and striped bars) or hMAb predigested with exogenous purified IgA1protease from H. influenzae Rd (gray bar), and washed. After 5 minutes at 37°C (black and gray bars) or 90 minute exposure of intact hMAb to endogenous protease produced by the S. pneumoniae organisms (striped bar), residual bound IgA1 was measured on washed bacteria stained with phycoerythrin-labeled mouse anti-human IgA1 by flow cytometry. Values for mean fluorescence intensity (MFI) ± standard deviation (SD) with intact MAb in 6 experiments are considered as 100%. P<.001 for exogenous and p<.02 for endogenous pneumococcal IgA1 protease compard with intact MAb. In Fig. 2B, 90 minute incubation of living wild-type S. pneumoniae (P210) resulted in loss of IgA1 (striped bar), but we observed no such loss of bound IgA1 from FITC-tagged dead organisms alone (black bar), or dead organisms which were washed, followed by addition of an equal number of unopsonized untagged wild-type live organisms (gray bar). The percent binding of IgA1 (Fcα), as determined by the MFI at 90 minutes, was determined by flow cytometry, as in 2A. Data are shown as the mean ±SD for 4 experiments. In Figure 2C, protease-deficient S. pneumoniae (strain P354) are incubated with intact or protease-treated IgA1 or IgA2 are incubated for 90 minutes, washed and surface Fca heavy chains and κ light chains are detectedy by flow cytometry. IgA1 protease decreases the amount of binding of IgA1 Fcα to S. pneumoniae (p=.007) but not the κ light chain binding, nor the Fcα which is resistant to the enzyme. Results are shown as mean of maximum binding (MFI) ±SD for 2 experiments.