Figure 1.
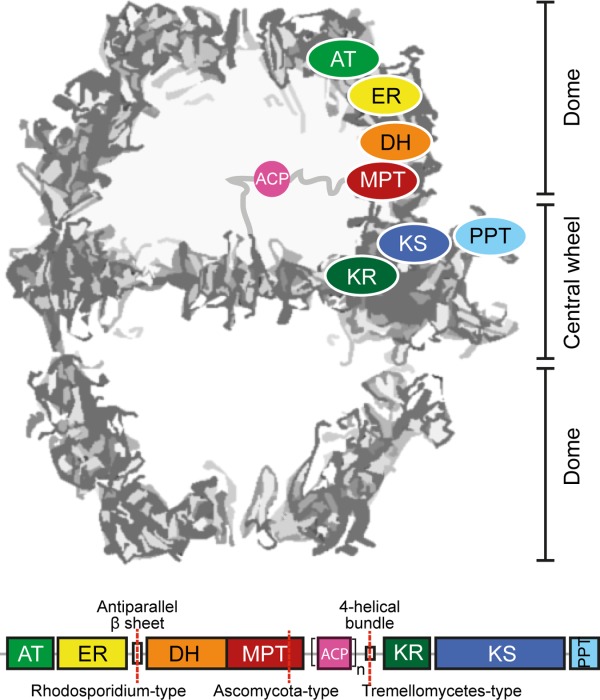
Architecture and domain organization of fungal FAS I. Fungal FAS I is an overall D3 symmetric barrel-shaped complex of homohexameric or heterodocameric (α6β6) oligomerization. For clarity of the structure representation, the protein is abstracted as a cross-section along the threefold axis. Fatty acid synthesis proceeds in two compartments, each of which is lined by three full sets of catalytic domains. The positioning of a set of domains is indicated for the upper compartment. The domains ketoacyl synthase (KS), ketoacyl reductase (KR), and phosphopantetheine transferase (PPT) comprise the central wheel part, while the other domains, acetyl-transferase (AT), enoyl reductase (ER), dehydratase (DH), and malonyl-palmitoyl-transferase (MPT) make up the dome-like structure. Fungal FAS I can be encoded by a single gene, as in the case of the group of Ustilaginomcetes, or split into two separate genes (FAS1 encoding the β-chain and FAS2 encoding the α-chain) as indicated. A mobile acyl carrier protein (ACP) as a single (n = 1) or duplicated domain (n = 2) spans the inner volume of the compartments, tethered by flexible linkers to the center of the wheel and the wall of the dome (abstracted by gray lines).
