Figure 3.
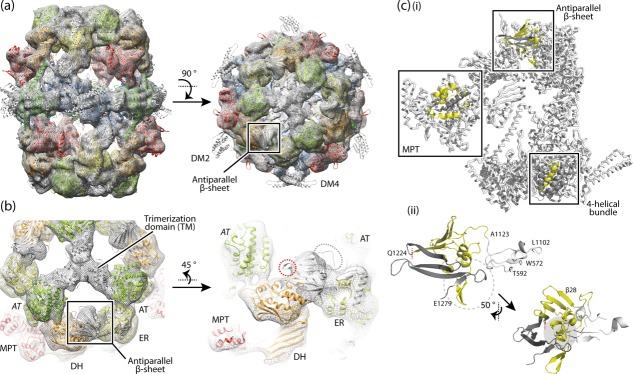
The structure of R. toruloides FAS I. (a) Cryo-EM map at 7.8 Å resolution with the X-ray structure of T. lanuginosus FAS I (pdb codes 2uva, 2uvb)11 fitted into the map as a rigid body. The protein is shown in side and top view. The polypeptides are colored according to the scheme above (see Fig. 1). Structural elements and the antiparallel β-sheet are highlighted. (b) 3D map and structural model as in (a) with zoom onto the antiparallel β-sheet domain. Domains are labeled. AT (italic letters) is provided from the neighboring polypeptide chain. The splitting site as occurring in R. toruloides FAS I is highlighted by a red circle; the loop connecting the β-sheet with the ER by a gray circle. (c, i) A single α-chain and a single β-chain are extracted from the fungal FAS I (T. lanuginosus FAS I; pdb codes 2uva, 2uvb)11 and splitting sites as occurring in fungal FAS I types Ascomycota (splitting site within MPT domain), Tremellomycetes (splitting site within 4-helical bundle) and Rhodosporidium (splitting site within antiparallel β-sheet) are indicated. In the highlighted structural elements, the β-chain part is shown in yellow and the α-chain part in dark gray. (ii) Antiparallel β-sheet composed of the β-chain and α-chain of R. toruloides FAS I; model and coloring as in (i); the left figure is roughly in the orientation of the side view in (a). The interface is comprised by formation of a curved β-sheet that interacts with α-helices at the concave face (highlighted by gray background), intertwined hairpin loops (highlighted by dashed circle), and the hairpin loop of α-chain interacting with a globular fold of the ER domain (colored in white). Residue numbers are given for defining borders (T. lanuginosus FAS I numbering).
