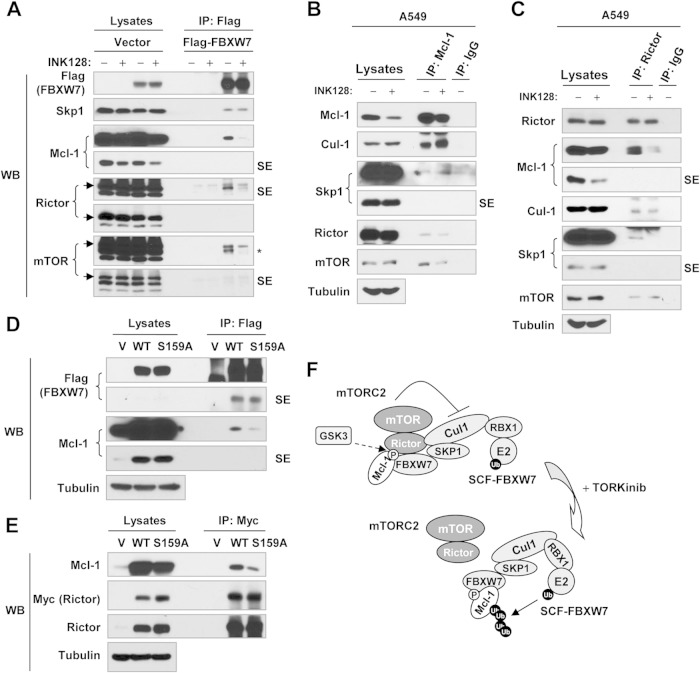
FIG 6.
Detection of a possible association between mTORC2 and the SCF-FBXW7 complex (A to E) and a working model of negative regulation of SCF-FBXW7-mediated Mcl-1 degradation by mTORC2 (F). (A) 293T cells were transfected with the vector or a Flag-FBXW7 plasmid, and after 48 h, they were treated with 100 nM INK128 for 2 h. (B and C) A549 cells were exposed to DMSO or 100 nM INK128 for 3 h. (D) 293T cells were cotransfected with Flag-FBXW7 and WT or mutant Mcl-1 for 48 h. (E) 293T cells were cotransfected with myc-rictor and WT or mutant Mcl-1 for 48 h. After the aforementioned transfections or treatment, the cells were harvested for preparation of whole-cell protein lysates and subsequent IP-Western blotting (WB) to detect the indicated proteins. SE, short exposure; V, vector; *, unstripped rictor band. (F) Working model of negative regulation of SCF-FBXW7-mediated Mcl-1 degradation by mTORC2. GSK3-dependent phosphorylation of Mcl-1 at Ser159 enhances the association of Mcl-1 with both the SCF-FBXW7 complex and mTORC2; inhibition of mTORC2 (e.g., with a TORKinib) facilitates the dissociation of mTORC2 from the SCF-FBXW7 complex, allowing the SCF-FBXW7 complex to degrade Mcl-1 by the ubiquitin (Ub)-proteasome (P) pathway.