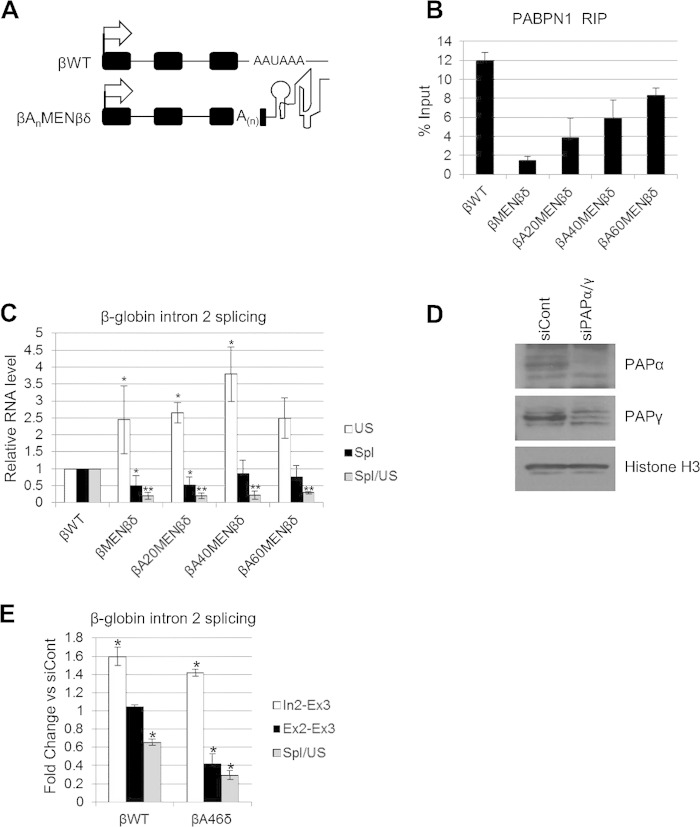
FIG 4.
An internal A-tract does not promote splicing in the absence of a 3′-terminal poly(A) tail. (A) Schematics of βWT and βAnMENβδ plasmids, with the positions of the internal A-tracts within exon 3 indicated. Other symbols are the same as in Fig. 1A. (B) RNA immunoprecipitation to assay the level of unspliced β-globin RNAs bound by PABPN1 in HeLa cells transiently transfected with the βWT, βMENβδ, βA20MENβδ, βA40MENβδ, or βA60MENβδ construct. Values are expressed as % input following normalization to PSMB3 pre-mRNA levels. (C) RT-qPCR analysis of β-globin exon 2 and 3 splicing in HeLa cells transiently transfected with the βWT, βMENβδ, βA20MENβδ, βA40MENβδ, or βA60MENβδ construct. The level of each RNA species was quantitated relative to that recovered from cells transfected with the βWT construct, which was given a value of 1. (D) Western blot analysis of PAPα and PAPγ proteins in cells treated with control or PAPα- and PAPγ-specific siRNAs. Histone H3 is shown as a loading control. (E) RT-qPCR analysis of exon 2 and 3 splicing in βWT and βA46δ cells treated with control or PAPα- and PAPγ-specific siRNAs. The level of each RNA species is shown as the fold change compared to the level in control siRNA-treated cells after normalization to GAPDH mRNA. All error bars represent standard deviations for at least three biological replicates. *, P < 0.05; **, P < 0.01. US, unspliced; Spl, spliced.