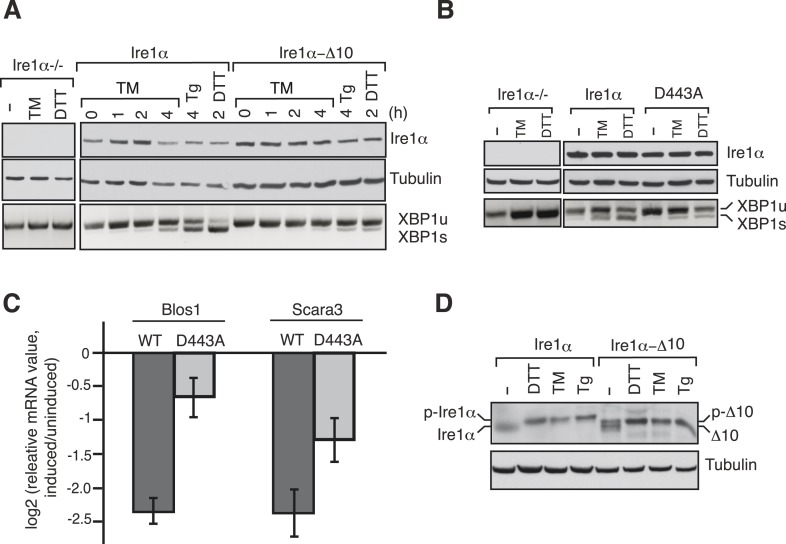
Figure 6. The Ire1α interaction with the Sec61 translocon ensures efficient cleavage of ER-targeted mRNAs.
(A) HEK 293 Ire1α−/− cells generated by CRISPR/Cas9 were stably complemented with Ire1α-HA or its mutant (Δ10). The expression of these constructs was controlled by doxycycline, but the cells were not induced with doxycyline in order to achieve low expression levels of Ire1α. Cells were harvested in Trizol after either treatment with tunicamycin (TM: 5 μg/ml), thapsigargin (Tg: 2.5 μg/ml) or DTT (10 mM) for the indicated time periods and analyzed by XBP1u mRNA splicing assay and IB with the indicated antibodies. (B) Mouse embryonic fibroblast (MEF) Ire1α−/− cells complemented with Ire1α-HA or its mutant (D443A) were harvested after either treatment with TM (5 μg/ml) for 5 hr or DTT (10 mM) for 2 hr and analyzed by XBP1u mRNA splicing assay and IB as described in Figure 2D. (C) The MEF Ire1α−/− cells complemented with indicated Ire1α variants were treated with TM (5 μg/ml) for 6 hr and analyzed by qPCR to measure Blos1 and Scara3 mRNA abundance. We normalized all mRNA abundance measurements to the housekeeping control Rpl19 mRNA. (D) HEK 293 Ire1α−/− cells stably expressing Ire1α-HA or its mutant (Δ10) were treated with DTT for 2 hr, TM for 5 hr, Tg for 5 hr and analyzed for phosphorylated Ire1α.