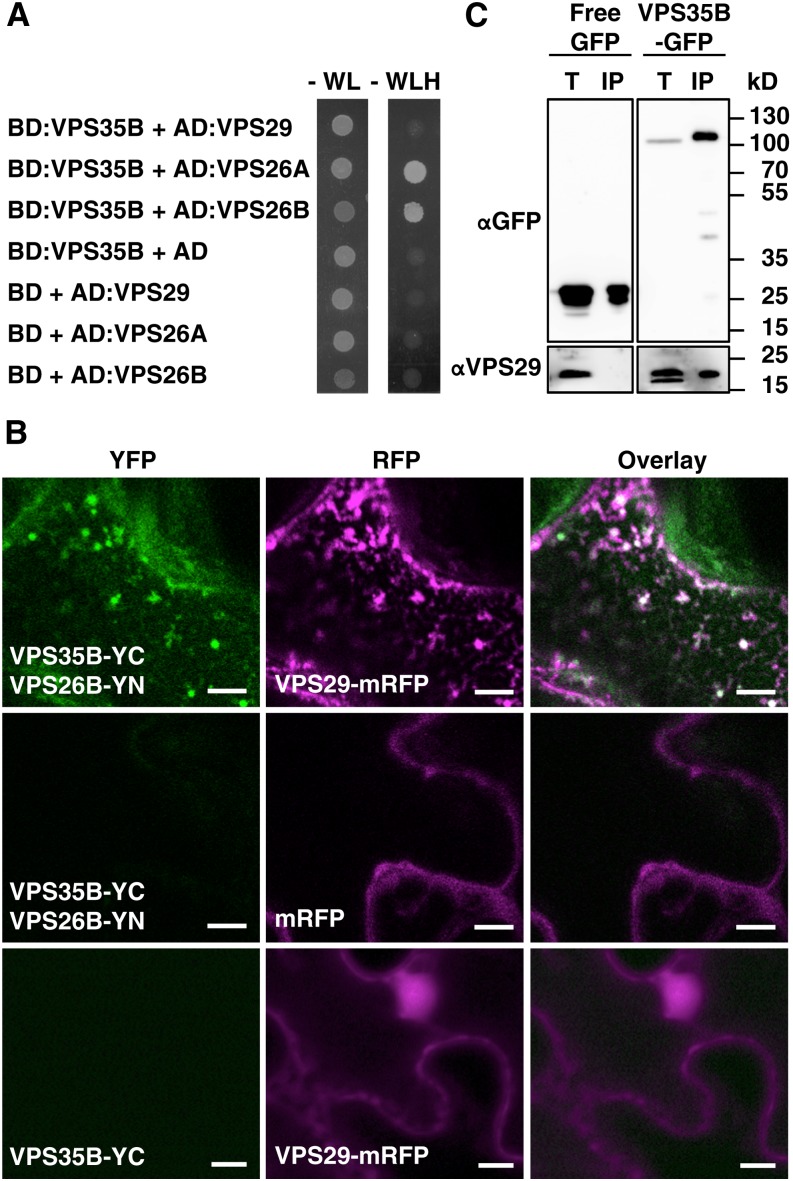
Figure 3.
VPS35B Interacts with Retromer Core Subunits.
(A) VPS35B interacts with VPS26A and VPS26B as indicated by the strong growth of yeast strains coexpressing VPS35B (bait) and VPS26A or VPS26B (prey) on selective medium (-WLH) after incubation for 3 d at 28°C. Only very weak growth is observed for VPS35B- and VPS29-expressing yeast cells. Control strains that expressed empty bait/prey vectors with the different retromer subunits show no growth under the same conditions. AD, activation domain; BD, binding domain.
(B) VPS29 is required for in vivo interaction of VPS35B and VPS26B. YFP fluorescence is detected in N. benthamiana leaves upon coexpression of VPS35B-YC and VPS26B-YN with VPS29-mRFP (top) but not with free mRFP (middle). No YFP signal is detected in infiltrated leaves expressing VPS35B-YC and VPS29-mRFP only (bottom). YC is the C-terminal portion of YFP, and YN is the N-terminal portion of YFP. Bars = 5 μm.
(C) Immunoblot analysis reveals the presence of VPS29 in VPS35B immunocomplexes. Total proteins from VPS35B-GFP- and free-GFP-expressing seedlings were extracted with salt-free buffer, and immunoprecipitation was performed with anti-GFP monoclonal antibody. Endogenous VPS29 (21 kD) was detected in total fractions (T) of both transgenic lines and coimmunoprecipitated (IP) with VPS35B-GFP (116 kD) but not with free GFP (27 kD). The total protein:immunoprecipitate ratio is 1:60. Immunoblots were probed with anti-GFP or anti-VPS29 antibody.