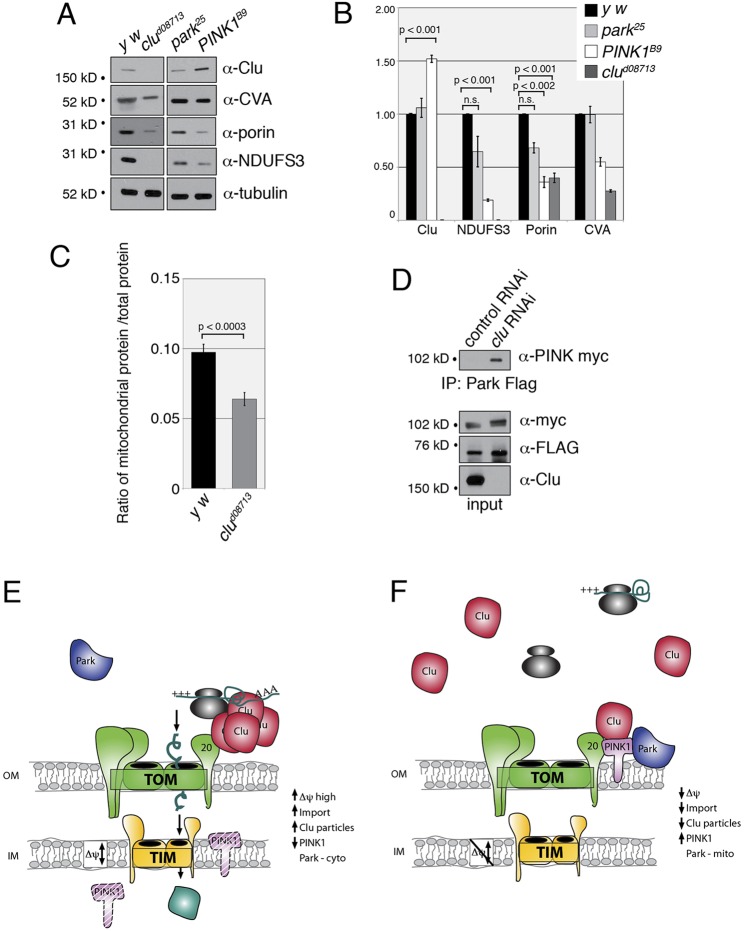
Fig. 7.
Lack of Clu decreases mitochondrial protein levels and causes PINK1 and Park to interact. (A) A representative western blot showing that levels of mitochondrial proteins are reduced in clu and PINK1B9 mutant adults, but are not substantially reduced in park mutants. (B) Quantification of mitochondrial proteins in park, PINK1B9 and clu mutant adults. Protein levels were normalized to tubulin for a loading control, then normalized to wild type (y w) for comparison. The error bars represent ±s.e.m. P-values were determined using Excel 2004 Toolpak and a two-tailed Student's t-test for each comparison. (C) In clu mutants, the ratio of mitochondrial protein to total protein is significantly smaller compared with wild type (y w). (D) PINK1-myc and Park-FLAG co-transfected S2R+ cells treated with control (luciferase) or clu RNAi show that PINK1 and Park do not form a complex under normal cell culture conditions in the presence of endogenous Clu (control RNAi lane), but are able to co-immunoprecipitate in the absence of Clu (clu RNAi lane). (E,F) A working model for Clu function. (E) Healthy mitochondria have associated Clu particles, undergo normal mitochondrial protein import and have low levels of PINK1. (F) When protein import is disrupted, Clu particles are lost, PINK1 is stabilized on the outer mitochondrial membrane and is thus able to recruit Park. Clu may or may not directly bind TOM20 or PINK1.