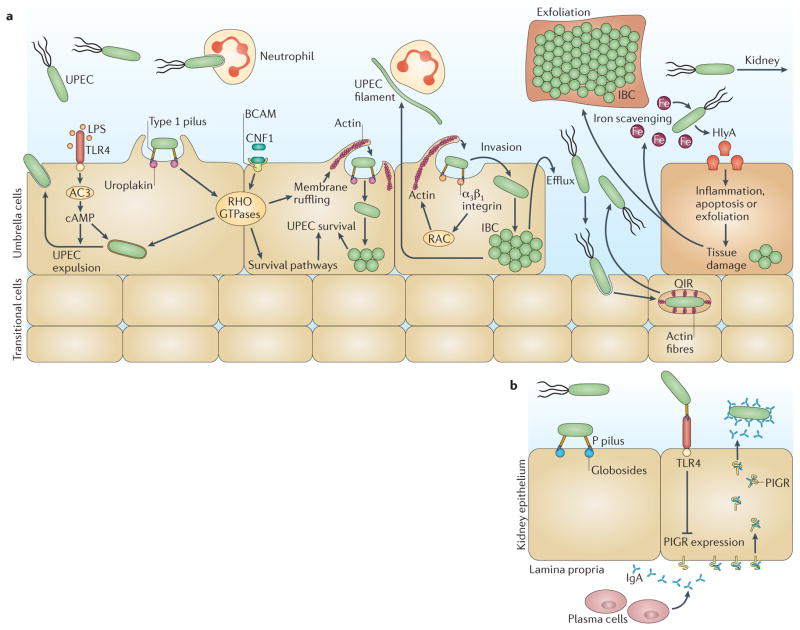
Figure 3. Virulence factors of uropathogenic Escherichia coli that contribute to urinary tract infections.
a | In the bladder, uropathogenic Escherichia coli (UPEC) expression of type 1 pili is essential for colonization, invasion and persistence. The type 1 pilus adhesin, FimH, binds mannosylated uroplakins and integrins that coat the surface of umbrella cells. Uroplakin binding by FimH induces actin rearrangement and bacterial internalization via unknown mechanisms. FimH–α3β1 integrin interactions induce actin rearrangement via activation of RHO-family GTPases (such as RAC proteins), resulting in bacterial invasion. Inside the host cell, UPEC can subvert host defences and resist antibiotic treatment. However, lipopolysaccharide (LPS) released by UPEC is sensed by Toll-like receptor 4 (TLR4), which induces cyclic AMP (cAMP) production via adenylyl cyclase 3 (AC3) activation, resulting in exocytosis of vesicular UPEC across the apical plasma membrane. UPEC subverts this innate defence mechanism by escaping into the cytoplasm, where it then multiplies to form intracellular bacterial communities (IBCs). Maturation of IBCs causes bacterial dispersal and allows the invasion of other host cells, which enables UPEC to re-enter the IBC cycle. Alternatively, UPEC can establish quiescent intracellular reservoirs (QIRs) in the underlying transitional cells. QIRs consist of 4–10 non-replicating bacteria within membrane-bound compartments encased in F-actin and can remain viable for months. In addition, UPEC survives within the harsh bladder environment by secreting several factors that are important for nutrient acquisition. The toxin α-haemolysin (HlyA) promotes host cell lysis through pore formation, facilitating iron release and nutrient acquisition. The siderophores expressed by UPEC allow the bacterium to scavenge iron and thus promote survival during a urinary tract infection (UTI). HlyA also triggers epithelial exfoliation to promote the spread of UPEC to other hosts following urine expulsion or to expose deeper layers of the uroepithelium for QIRs. Cytotoxic necrotizing factor 1 (CNF1) is also important for host cell remodelling and functions by binding to the receptor basal cell adhesion molecule (BCAM) on host cells to induce constitutive activation of the RHO GTPases RAC1, RHOA and cell division control 42 (CDC42), resulting in actin cytoskeletal rearrangements and membrane ruffling. Activation of RAC1 also induces the host cell anti-apoptotic and pro-survival pathways, preventing apoptosis of colonized epithelial cells and allowing the UPEC population to expand. The extracellular survival of UPEC also requires evasion of the innate immune system by the adoption of a filamentous morphology, which renders the bacterium more resistant to neutrophil killing than their bacillary form. b | UPEC colonization of the kidneys is dependent on expression of pyelonephritis-associated (P) pili, which bind globoside-containing glycolipids lining the renal tissue. The P pilus adhesin, PapG, also interacts with TLR4, reducing the expression of polymeric immunoglobulin receptor (PIGR). This results in impaired immunoglobulin A (IgA) transport across the epithelium, thereby modulating the local secretory antibody immune response and preventing UPEC opsonization and clearance.