Abstract
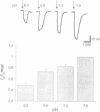
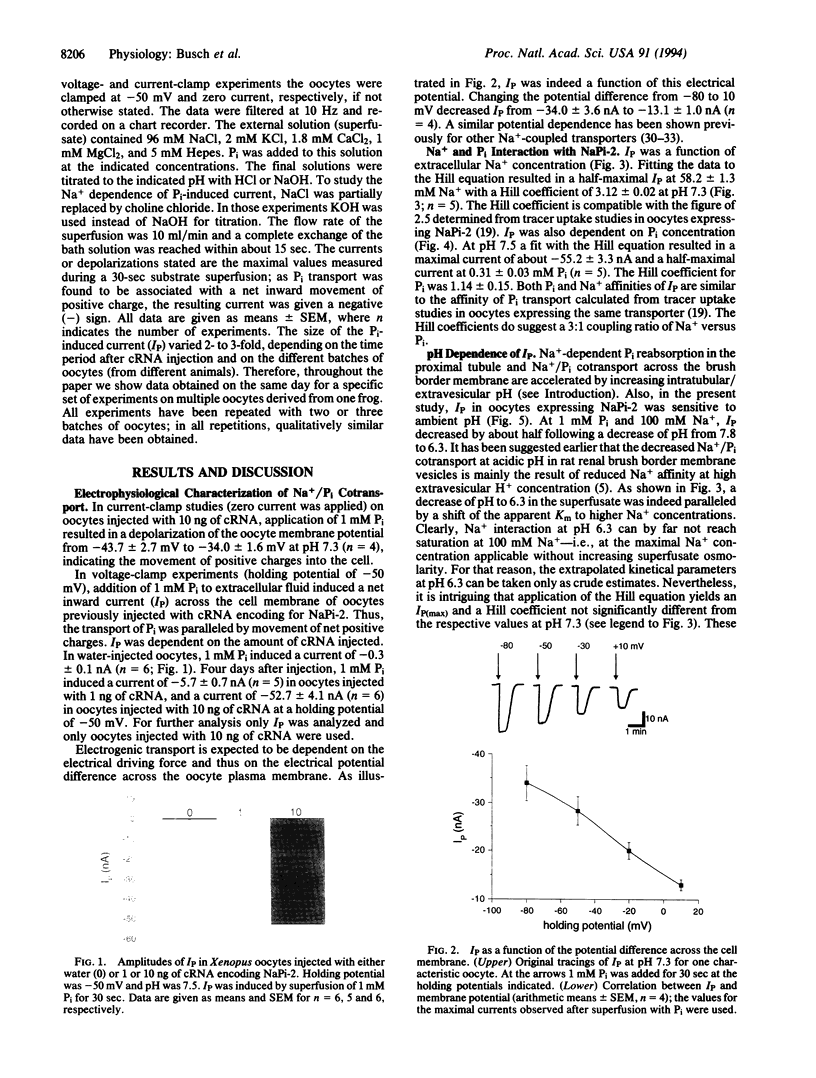
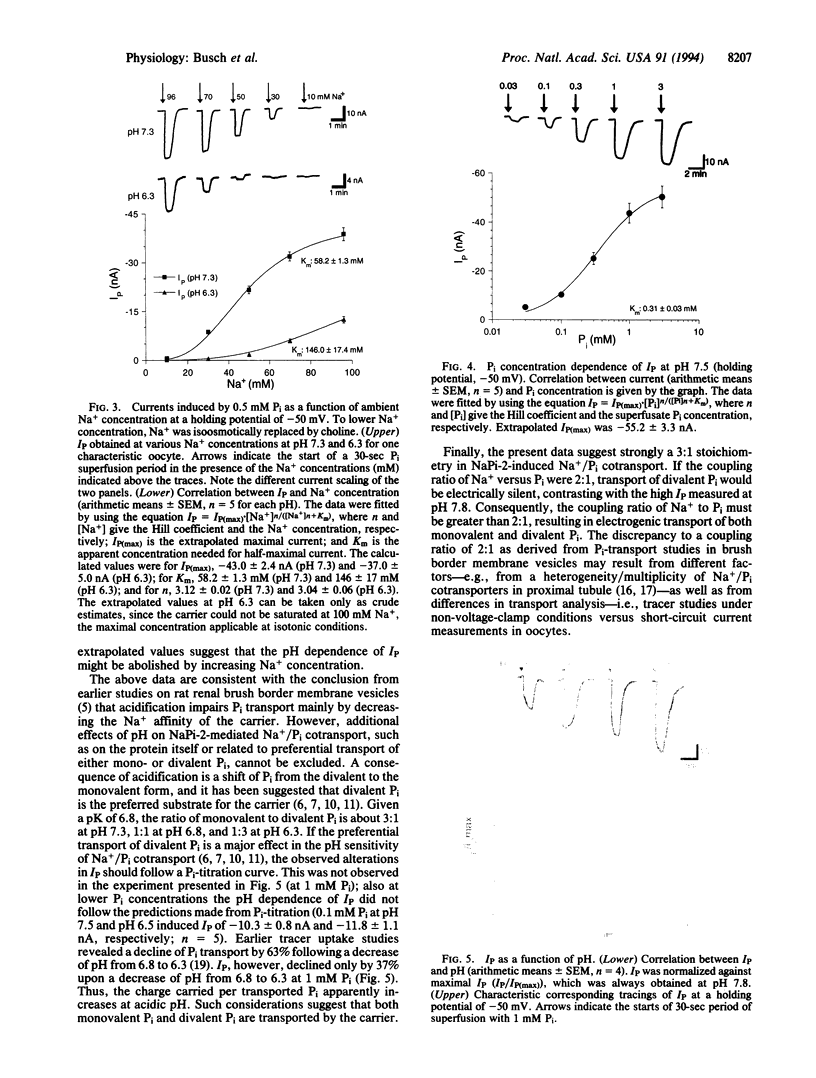
Phosphate (Pi) reabsorption in renal proximal tubules involves Na+/Pi cotransport across the brush border membrane; its transport rate is influenced by the Na(+)-coupled transport of other solutes as well as by pH. In the present study, we have expressed a cloned rat renal brush border membrane Na+/Pi cotransporter (NaPi-2) in Xenopus laevis oocytes and have analyzed its electrophysiologic properties in voltage- and current-clamp studies. Addition of Pi to Na(+)-containing superfusates resulted in a depolarization of the membrane potential and, in voltage-clamped oocytes, in an inward current (IP). An analysis of the Na+ and/or Pi concentration dependence of IP suggested a Na+/Pi stoichiometry of 3:1. IP was increased by increasing the pH of the superfusate; this phenomenon seems to be mainly related to a lowering of the affinity for Na+ interaction by increasing H+ concentration. The present data suggest that known properties of Pi handling at the tubular/membrane level are "directly" related to specific characteristics of the transport molecule (NaPi-2) involved.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstutz M., Mohrmann M., Gmaj P., Murer H. Effect of pH on phosphate transport in rat renal brush border membrane vesicles. Am J Physiol. 1985 May;248(5 Pt 2):F705–F710. doi: 10.1152/ajprenal.1985.248.5.F705. [DOI] [PubMed] [Google Scholar]
- Barrett P. Q., Aronson P. S. Glucose and alanine inhibition of phosphate transport in renal microvillus membrane vesicles. Am J Physiol. 1982 Feb;242(2):F126–F131. doi: 10.1152/ajprenal.1982.242.2.F126. [DOI] [PubMed] [Google Scholar]
- Biber J., Caderas G., Stange G., Werner A., Murer H. Effect of low-phosphate diet on sodium/phosphate cotransport mRNA and protein content and on oocyte expression of phosphate transport. Pediatr Nephrol. 1993 Dec;7(6):823–826. doi: 10.1007/BF01213368. [DOI] [PubMed] [Google Scholar]
- Biber J., Custer M., Werner A., Kaissling B., Murer H. Localization of NaPi-1, a Na/Pi cotransporter, in rabbit kidney proximal tubules. II. Localization by immunohistochemistry. Pflugers Arch. 1993 Aug;424(3-4):210–215. doi: 10.1007/BF00384344. [DOI] [PubMed] [Google Scholar]
- Bindels R. J., van den Broek L. A., van Os C. H. Effect of pH on the kinetics of Na+-dependent phosphate transport in rat renal brush-border membranes. Biochim Biophys Acta. 1987 Feb 12;897(1):83–92. doi: 10.1016/0005-2736(87)90317-8. [DOI] [PubMed] [Google Scholar]
- Burckhardt G., Stern H., Murer H. The influence of pH on phosphate transport into rat renal brush border membrane vesicles. Pflugers Arch. 1981 May;390(2):191–197. doi: 10.1007/BF00590206. [DOI] [PubMed] [Google Scholar]
- Busch A. E., Kavanaugh M. P., Varnum M. D., Adelman J. P., North R. A. Regulation by second messengers of the slowly activating, voltage-dependent potassium current expressed in Xenopus oocytes. J Physiol. 1992 May;450:491–502. doi: 10.1113/jphysiol.1992.sp019138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béliveau R., Strevey J. The sodium gradient induces conformational changes in the renal phosphate carrier. J Biol Chem. 1987 Dec 15;262(35):16885–16891. [PubMed] [Google Scholar]
- Béliveau R., Strévey J. Kinetic model for phosphate transport in renal brush-border membranes. Am J Physiol. 1988 Mar;254(3 Pt 2):F329–F336. doi: 10.1152/ajprenal.1988.254.3.F329. [DOI] [PubMed] [Google Scholar]
- Béliveau R., Strévey J. Phosphate transport in kidneys: effect of transmembrane electrical potential. Am J Physiol. 1991 Oct;261(4 Pt 2):F663–F669. doi: 10.1152/ajprenal.1991.261.4.F663. [DOI] [PubMed] [Google Scholar]
- Cheng L., Sacktor B. Sodium gradient-dependent phosphate transport in renal brush border membrane vesicles. J Biol Chem. 1981 Feb 25;256(4):1556–1564. [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Custer M., Lötscher M., Biber J., Murer H., Kaissling B. Expression of Na-P(i) cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am J Physiol. 1994 May;266(5 Pt 2):F767–F774. doi: 10.1152/ajprenal.1994.266.5.F767. [DOI] [PubMed] [Google Scholar]
- Custer M., Meier F., Schlatter E., Greger R., Garcia-Perez A., Biber J., Murer H. Localization of NaPi-1, a Na-Pi cotransporter, in rabbit kidney proximal tubules. I. mRNA localization by reverse transcription/polymerase chain reaction. Pflugers Arch. 1993 Aug;424(3-4):203–209. doi: 10.1007/BF00384343. [DOI] [PubMed] [Google Scholar]
- Danisi G., Murer H., Straub R. W. Effect of pH on phosphate transport into intestinal brush-border membrane vesicles. Am J Physiol. 1984 Feb;246(2 Pt 1):G180–G186. doi: 10.1152/ajpgi.1984.246.2.G180. [DOI] [PubMed] [Google Scholar]
- Dennis V. W., Brazy P. C. Sodium, phosphate, glucose, bicarbonate, and alanine interactions in the isolated proximal convoluted tubule of the rabbit kidney. J Clin Invest. 1978 Aug;62(2):387–397. doi: 10.1172/JCI109140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmaj P., Murer H. Cellular mechanisms of inorganic phosphate transport in kidney. Physiol Rev. 1986 Jan;66(1):36–70. doi: 10.1152/physrev.1986.66.1.36. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P. Voltage dependence of facilitated arginine flux mediated by the system y+ basic amino acid transporter. Biochemistry. 1993 Jun 8;32(22):5781–5785. doi: 10.1021/bi00073a009. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Storck T., Conradt M., Stoffel W. Electrogenic L-glutamate uptake in Xenopus laevis oocytes expressing a cloned rat brain L-glutamate/L-aspartate transporter (GLAST-1). J Biol Chem. 1993 Jul 15;268(20):14594–14596. [PubMed] [Google Scholar]
- Levi M. Heterogeneity of Pi transport by BBM from superficial and juxtamedullary cortex of rat. Am J Physiol. 1990 Jun;258(6 Pt 2):F1616–F1624. doi: 10.1152/ajprenal.1990.258.6.F1616. [DOI] [PubMed] [Google Scholar]
- Magagnin S., Werner A., Markovich D., Sorribas V., Stange G., Biber J., Murer H. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5979–5983. doi: 10.1073/pnas.90.13.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovich D., Stange G., Bertran J., Palacin M., Werner A., Biber J., Murer H. Two mRNA transcripts (rBAT-1 and rBAT-2) are involved in system b0,(+)-related amino acid transport. J Biol Chem. 1993 Jan 15;268(2):1362–1367. [PubMed] [Google Scholar]
- Murer H. Homer Smith Award. Cellular mechanisms in proximal tubular Pi reabsorption: some answers and more questions. J Am Soc Nephrol. 1992 Jun;2(12):1649–1665. doi: 10.1681/ASN.V2121649. [DOI] [PubMed] [Google Scholar]
- Parent L., Supplisson S., Loo D. D., Wright E. M. Electrogenic properties of the cloned Na+/glucose cotransporter: I. Voltage-clamp studies. J Membr Biol. 1992 Jan;125(1):49–62. doi: 10.1007/BF00235797. [DOI] [PubMed] [Google Scholar]
- Parent L., Supplisson S., Loo D. D., Wright E. M. Electrogenic properties of the cloned Na+/glucose cotransporter: II. A transport model under nonrapid equilibrium conditions. J Membr Biol. 1992 Jan;125(1):63–79. doi: 10.1007/BF00235798. [DOI] [PubMed] [Google Scholar]
- Sacktor B., Cheng L. Sodium gradient-dependent phosphate transport in renal brush border membrane vesicles. Effect of an intravesicular greater than extravesicular proton gradient. J Biol Chem. 1981 Aug 10;256(15):8080–8084. [PubMed] [Google Scholar]
- Strévey J., Giroux S., Béliveau R. pH gradient as an additional driving force in the renal re-absorption of phosphate. Biochem J. 1990 Nov 1;271(3):687–692. doi: 10.1042/bj2710687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenhouse H. S., Werner A., Biber J., Ma S., Martel J., Roy S., Murer H. Renal Na(+)-phosphate cotransport in murine X-linked hypophosphatemic rickets. Molecular characterization. J Clin Invest. 1994 Feb;93(2):671–676. doi: 10.1172/JCI117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. T., Dousa T. P. Phosphate transport by brushborder membranes from superficial and juxtamedullary cortex. Kidney Int. 1985 Jun;27(6):879–885. doi: 10.1038/ki.1985.95. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Phosphate transport in the proximal convolution of the rat kidney. III. Effect of extracellular and intracellular pH. Pflugers Arch. 1978 Oct 18;377(1):33–42. doi: 10.1007/BF00584371. [DOI] [PubMed] [Google Scholar]
- Werner A., Kempson S. A., Biber J., Murer H. Increase of Na/Pi-cotransport encoding mRNA in response to low Pi diet in rat kidney cortex. J Biol Chem. 1994 Mar 4;269(9):6637–6639. [PubMed] [Google Scholar]
- Werner A., Moore M. L., Mantei N., Biber J., Semenza G., Murer H. Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9608–9612. doi: 10.1073/pnas.88.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]