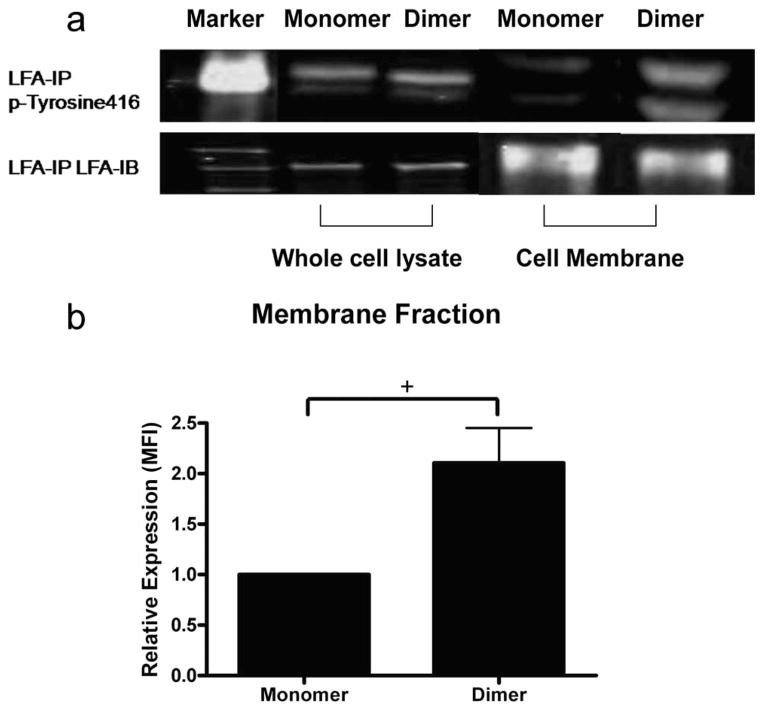
FIGURE 6.
Phospho-SFK (Tyr416) associates with LFA-1 upon adhesion to ICAM-1. Murine neutrophils (2 × 107) were allowed to adhere on monomeric or dimeric ICAM-1 and incubated for 7 min before cell lysis. LFA-1 was immunoprecipitated (IP) with polyclonal antisera and subjected to immunoblotting (IB) with antiphospho-SFK. Murine neutrophils (2 × 107) adherent to monomer or dimer were then frozen in liquid nitrogen. Cell membrane preparations were prepared as described in Materials and Methods. Samples of each preparation were immunoprecipitated with anti-LFA. a, Phospho-SFK (Tyr416) associated with LFA-1 on monomer and dimer in whole-cell lysate and membrane fraction (upper blot). The two bands coincide with 55- and 58-kDa markers and represent the SFK kinase Lyn, which migrates as a doublet at this molecular mass. b, Quantitative image analysis of the immunoprecipitated Tyr416 was performed for the membrane fraction of neutrophils adherent to monomeric vs dimeric ICAM-1. Bars represent normalized intensity of phospho-SFK signal associating with PMN membrane adherent to monomer vs dimer ICAM-1 from three experiments. +, p < 0.05.